Case No: A3 2016 4260
Neutral Citation Number: [2018] EWCA Civ 12
IN THE COURT OF APPEAL (CIVIL DIVISION)
ON APPEAL FROM THE HIGH COURT, CHANCERY DIVISION
PATENTS COURT
MR JUSTICE HENRY CARR
[2016] EWHC 1285 (Pat)
Royal Courts of Justice
Strand, London, WC2A 2LL
Date: 18 January 2018
Before :
LORD JUSTICE LEWISON
and
LORD JUSTICE KITCHIN
– – – – – – – – – – – – – – – – – – – – –
Between :
| Hospira UK Limited | Claimant/
Respondent |
|
| – and – | ||
| Cubist Pharmaceuticals LLC | Defendant/Appellant |
– – – – – – – – – – – – – – – – – – – – –
Thomas Hinchliffe QC and Stuart Baran (instructed by Carpmaels & Ransford LLP) for the Appellant
Richard Meade QC and Isabel Jamal (instructed by Taylor Wessing LLP) for the Respondent
Hearing dates: 12 December 2017
– – – – – – – – – – – – – – – – – – – – –
Judgment
Lord Justice Kitchin:
1. This appeal concerns a method of purification of the antibiotic daptomycin. Although at trial the judge, Henry Carr J, was concerned with multiple issues on several patents, the sole issue on this appeal is whether the method claimed in European Patent (UK) 2,264,047 (“the patent”) is obvious over an article in the journal Biotechnology Techniques by Sung-Chyr Lin and Horng-Jyh Jiang entitled “Recovery and Purification of the lipopeptide biosurfactant of Bacillus subtilis by ultrafiltration” (“Lin and Jiang”). In his decision dated 10 June 2016 the judge held that the patent was invalid and on 17 October 2016 he made an order for its revocation. The proprietor of the patent, Cubist Pharmaceuticals LLC (“Cubist”), now appeals against that decision and order.
2. Daptomycin is a useful and commercially successful antibiotic. It is effective against certain Gram-positive bacteria which can cause serious infections, including MRSA-borne endocarditis. It was originally developed by Eli Lilly (“Lilly”) but was abandoned, at least in part because of the limitations of the purification process that Lilly was using. The purity of a compound is clearly of great importance if it is to be administered to humans as a medicine and Lilly thought that daptomycin could only be produced with a purity of 90-93% and at a relatively low yield.
3. The patent describes a new way to purify daptomycin which relies upon the ability of daptomycin molecules under particular conditions to form into structures called micelles and then, under different conditions, to dissociate. This permits the use of a two stage purification process in which impurities, whether smaller or larger than the daptomycin molecules, are removed. Before explaining how this process works and why the judge found it to be obvious, I must say a little about the technical background.
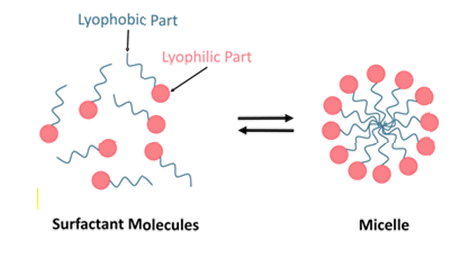
4. Daptomycin is a surfactant, that is to say a compound that tends to lower the surface tension between two liquids or between a liquid and a solid. A biological surfactant is called a biosurfactant. Each molecule of a surfactant has a characteristic structure with a lyophilic (solvent loving) and a lyophobic (solvent hating) part. When such molecules are present in a solvent at a sufficient concentration (called the critical micelle concentration or CMC) they gather together with their lyophobic parts facing inwards and their lyophilic parts facing outwards in such a way as to form spherical aggregates or micelles. The judge illustrated this with a diagram which I reproduce below:
6. Daptomycin is also a lipopeptide. Lipopeptides are molecules consisting of a lipid (such as a fat) connected to a peptide (a short chain of amino acids). The peptide portion is hydrophilic and the lipid portion is hydrophobic. The judge found that it was widely known that lipopeptides as a class were biosurfactants although he also accepted that the skilled person could not be certain that any individual lipopeptide was a surfactant without testing it.
7. There were various standard tests at the priority date to determine whether a molecule was a surfactant. Each was easy to perform. One was the “shake test”. A surfactant will produce a stable foam on shaking, whereas a non-surfactant will either not produce a foam, or will produce a transient foam that quickly disappears. Another was described by Professor Myerson, the expert witness for Cubist. He said that his preferred approach was to measure the CMC of a fluid by electrical conductivity, and this would at the same time establish whether it was a surfactant. Both of these tests were common general knowledge at the priority date and could be performed simply in any reasonably equipped laboratory.
8. There was a dispute as to whether it was common general knowledge that daptomycin was a surfactant. It was known to be a lipopeptide, it has a hydrophilic head and a hydrophobic tail and Dr Baker, the expert witness for the claimant (“Hospira”), said it has a primary structure which is and was even more suggestive of it being a surfactant than that of a well-known biosurfactant called surfactin. The judge accepted this evidence. He found that the skilled team would not have been certain that daptomycin was a biosurfactant but, given its primary structure, they would have had a strong expectation that it was. This could have been confirmed by any of the simple tests.
9. I come next to the patent. The specification explains that the invention relates to a process for preparing a highly purified form of daptomycin which involves altering the pH of a daptomycin solution to change its CMC. The judge fairly explained the process in these terms:
“350. … The daptomycin is first formed into micelles by lowering the pH. The solution is then passed through an ultrafiltration membrane. The daptomycin micelles are retained on the filter, but smaller impurities pass through. Then the daptomycin that was retained on the membrane has its pH adjusted upwards to pH 6.5, causing the daptomycin micelles to break apart. The solution is passed through another ultrafiltration step. This time, the daptomycin monomers are able to pass through the filter, but larger impurities are retained on the filter, thereby separating them from the daptomycin.”
10. Claim 1, the only claim said to be independently valid, is as follows:
“1. A method for purifying daptomycin comprising:
(a) subjecting the daptomycin to conditions in which a daptomycin micellar solution is formed by altering the pH;
(b) separating the daptomycin micelles in the daptomycin micellar solution from low molecular weight contaminants by a size separation technique;
(c) subjecting the daptomycin to conditions in which a daptomycin monomeric solution is formed by altering the pH; and
(d) separating the monomeric daptomycin molecules in the daptomycin monomeric solution from high molecular weight molecules or aggregates by a size separation technique.”
11. The difficulty facing Cubist, however, was that the general idea of harnessing the ability to adjust the CMC of a surfactant solution in a process of purification had already been disclosed in Lin and Jiang, although the surfactant being purified there was surfactin and the adjustment of the CMC was achieved by adding methanol. The judge summarised the disclosure in this way:
“354. Lin & Jiang describe a process for purifying surfactin …. First, they formed micelles and removed certain low molecular weight impurities via ultrafiltration. Lin & Jiang then removed high molecular weight impurities that were retained on the ultrafiltration membrane together with the surfactin micelles by adding methanol to the ultrafiltration membrane to break the micelles into monomers, such that the monomeric surfactin passed through the filter, while the high molecular weight impurities were retained on the filter.
12. Lin and Jiang concludes with this more general teaching:
“This process can be further modified and employed for the recovery and purification of most surfactants from aqueous solutions at concentrations above the critical micelle concentration.”
13. The judge identified the differences between claim 1 of the patent and Lin and Jiang entirely correctly as being first, that Lin and Jiang is concerned with the purification of surfactin rather than daptomycin and secondly, that Lin and Jiang uses the addition of methanol rather than the alteration of pH to adjust the CMC and so dissociate the micelles in its second step.
14. Hospira contended that it would have been perfectly obvious to the skilled team that the purification method described in Lin and Jiang could be used for the purification of daptomycin, and it would have been an entirely routine matter for the team to use the alteration of pH rather than the addition of methanol to adjust the CMC. Cubist responded that it would not have been obvious to the skilled team that the Lin and Jiang method could be applied successfully to the purification of daptomycin because the team would not have expected the method to remove pyrogens, an impurity associated with daptomycin; the team would not have thought it likely that daptomycin was a surfactant which would form micelles; and it would not have been obvious to the team to use pH instead of methanol to adjust the CMC.
15. The judge concluded that claim 1 was obvious in the light of Lin and Jiang. His reasoning ran as follows. First, although Lin and Jiang describes a method of purifying surfactin, the skilled team would have appreciated that this method could be used more generally. Indeed, the authors expressly state that their method can be modified and employed for the recovery and purification of most surfactants from aqueous solutions at concentrations above the CMC.
16. Secondly, the skilled team would have appreciated that the method of Lin and Jiang would not achieve “total” purification and that pyrogens might remain but would have thought these impurities could be removed by standard techniques. The same was true of the method of the patent.
17. Thirdly, the skilled team would have appreciated that the method of Lin and Jiang was of potential relevance to the purification of daptomycin. The team would have had a real expectation, founded upon daptomycin’s primary structure and the fact that it was a lipopeptide, that it was a biosurfactant which would form micelles, and this could have been confirmed by simple tests.
18. Fourthly, the skilled team would have regarded the method described in Lin and Jiang as being workable for the purification of biosurfactants on a laboratory scale. The team would have appreciated that the first step of forming micelles and using ultrafiltration to remove the smaller impurities would have been thought useful as it was. But the team would have appreciated straight away that methanol should not be used in the second step to manipulate the CMC and disassociate the micelles for anything other than proof of concept. This was so for two reasons: first, methanol is toxic so it has to be removed to a very low level; and secondly, the Lin and Jiang method uses a lot of methanol, and this would lead to problems of solvent recovery at the end of the process.
19. Fifthly, once the skilled team had decided to use an alternative way to manipulate the CMC to dissociate the micelles, it was clear that control of pH was one of a short list of standard ways of doing this. Dr Baker thought that changing the pH and adjusting the temperature would have been thought of as particularly good ways of manipulating the CMC because they were readily controllable. Professor Myerson considered the team would have thought these methods were advantageous because they required fewer or no additions to the solution.
20. The judge therefore found that it would have been obvious to the skilled team to use the Lin and Jiang process for the purification of daptomycin but to use pH to adjust the CMC. It followed that the patent was invalid for lack of inventive step.
21. Upon this appeal there has been no real dispute between the parties as to the relevant legal principles. They are set out in the decisions of the Court of Appeal in MedImmune v Novartis Pharmaceuticals UK Ltd [2012] EWCA Civ 1234, [2013] RPC 27 at [84] to [94] and Hospira v Genentech [2016] EWCA Civ 780, [2017] RPC 13 at [9] to [13]. Cubist contends instead that the judge fell into error in the way he applied those principles to the facts of the case. It is argued that he failed properly to assess whether the skilled team would have considered that:
i) daptomycin was likely to be a surfactant which would form micelles; and
ii) the method of forming and breaking micelles disclosed in Lin and Jiang could be successfully replaced by a different method involving changing the pH.
22. I will deal with these contentions in turn.
Issue (i): appreciation that daptomycin was likely to be a surfactant which would form micelles
23. Cubist contends that the judge was not entitled to find that the skilled team would have had a real expectation that daptomycin was likely to be a surfactant which would form micelles, or that the team would have been able to confirm that by straightforward routine testing.
24. I accept that this is an important issue. Daptomycin is not mentioned in Lin and Jiang and so the obviousness case depends on whether the skilled team would have appreciated that it was likely to be a surfactant which would form micelles at concentrations above the CMC, and whether the team would have been able to confirm that appreciation by straightforward testing. If the skilled team would not have had such an appreciation or would not have been able to confirm it then it could hardly have been obvious to apply the Lin and Jiang purification process to daptomycin.
25. Here counsel for Cubist, Mr Thomas Hinchliffe QC, points to the judge’s finding that although the skilled team would have had a strong expectation that daptomycin was a surfactant, they could not have been sure that it was without testing it. However, Mr Hinchliffe continues, the judge had no basis for his further finding that any of the standard tests the team would carry out would confirm that expectation.
26. Mr Hinchliffe argues that the evidence on testing fell into two categories: first, evidence given by Dr Baker about testing for the formation of a stable foam, either during fermentation or on shaking a sample; and secondly, evidence given by Professor Myerson in cross examination that the CMC could be determined by electrical conductivity testing. He submits that neither provided the necessary basis for the judge’s finding of obviousness.
27. I will deal with each of these categories in turn but before doing so I must say a little more about the foundation for the judge’s finding that the skilled team would have had a strong expectation that daptomycin was a surfactant. In this regard the judge found first, that it was common general knowledge that lipopeptides as a class were biosurfactants, and that this was amply demonstrated by the literature. Secondly, there was no example in the evidence of any lipopeptide which was not a biosurfactant. And thirdly, the primary structure of a lipopeptide (which was possessed by daptomycin) was in and of itself sufficient to make a reasonable prediction that it would act as a surfactant. It was not seriously suggested that these findings were not properly founded in the evidence but in any event I am satisfied that they were. I am also in no doubt that they provided a proper basis for the judge’s finding that the skilled team would have had a real expectation that daptomycin was likely to be a surfactant. As a surfactant, it would form micelles under appropriate conditions.
28. I come next to the question whether standard tests would have confirmed that expectation and begin with the stable foam or shake test. Mr Hinchliffe has developed his submission as follows. He begins with the evidence of Dr Baker in chief. Dr Baker said that the skilled person would test whether daptomycin was a surfactant by checking for the presence of a stable foam either during fermentation or on shaking. In relation to the former, Dr Baker accepted in cross examination that because the fermentation broth was a complex mixture, this would not give any indication as to what was producing the foam. Thus, Mr Hinchliffe continues, only the shaking test could reliably be used to determine whether or not daptomycin was a surfactant. As to this, it is notable that Hospira did not perform any experiment to show what happens when daptomycin is shaken, despite provision having been made for experiments at the case management conference.
29. Moreover, submits Mr Hinchliffe, the judge heard direct evidence about the results of shaking a sample from Dr Kelleher, a witness of fact for Cubist. In the ordinary course of his work for Cubist he shook samples of daptomycin which had thawed following removal from the freezer but did not see any foam. Accordingly, argues Mr Hinchliffe, the judge had no evidence upon which to find that a shake test would have confirmed that daptomycin was a surfactant.
30. The judge did not doubt the veracity of Dr Kelleher’s evidence but found it of limited or no assistance for three reasons: (i) Cubist used a de-foaming agent in its fermentation broth, and so it was unsurprising that Dr Kelleher had not seen any foam; (ii) Dr Kelleher was unable to give details of the containers which he used; and (iii) the purpose of Dr Kelleher’s shaking was to make the mixture homogenous, and here it was important to avoid excessive shaking for this might denature the protein. Such considerations would not have applied to the “shake test”, the object of which would have been to see whether a strong stable foam could be created.
31. Mr Hinchliffe submits the judge was wrong to treat Dr Kelleher’s evidence in the way that he did, and that he had no proper reason to find that Dr Kelleher had not shaken his samples hard enough. He continues that the only reliable evidence before the court as to what happens when a sample of daptomycin is shaken was that of Dr Kelleher and he gave evidence that no foam was formed. In light of this the judge ought to have held that the skilled person performing the shake test would have concluded that daptomycin was not a surfactant.
32. I am not persuaded by any of these submissions. Important and clear evidence on this issue was given by Dr Baker. He said the shake test is a straightforward way to test whether a compound which is suspected of being a surfactant really is one. The technician simply has to put a pure solution of the compound into a test tube and shake it vigorously. If it is a surfactant, shaking will produce a stable foam. This routine test was well known to those working in the field at the priority date. We have been taken to the transcript of Dr Baker’s cross examination on this issue and he maintained the position he had taken in his reports. In his words: “the shaking will produce a stable foam if the compound is a surfactant”. Professor Myerson did not really take issue with this evidence. In his second report he simply said that he did not necessarily agree that the presence of foaming would have been “a conclusive indicator” that daptomycin was a surfactant, apparently because such foaming might be caused by impurities.
33. As for Dr Kelleher, he was a witness of fact and one of the named inventors of the patent. He became involved in the daptomycin project at the time that Cubist was seeking to scale up production and explained that he frequently shook samples which had been taken from the manufacturing process and stored in a freezer. When he or one of his colleagues wished to use a sample in an experiment for quality control purposes, it was thawed and shaken to ensure its homogeneity. He never saw a stable foam.
34. I am satisfied that the judge was entitled to find Dr Kelleher’s evidence of little or no value in determining what would happen in a properly conducted shake test. The fundamental problem with that evidence is that Dr Kelleher carried out his tests on samples which contained anti-foam. In addition, his tests were carried out for a different purpose and under other conditions the precise details of which he understandably could no longer recall.
35. In light of the foregoing, I have no doubt that the judge was entitled to hold on the evidence before him that a properly conducted shake test would have revealed that daptomycin was a surfactant. The critical evidence was that of Dr Baker to which there was no effective challenge. In these circumstances there was no need for Hospira to carry out any experiment to establish this aspect of its case.
36. That brings me to the judge’s further finding that an electrical conductivity test would also have confirmed that daptomycin was a surfactant. In this test the change in conductivity of a solution of a material is measured as the concentration of the solution is increased (or another relevant condition such as pH is changed), and the CMC is identified by an inflection in the curve.
37. The possibility of determining whether daptomycin was a surfactant by carrying out a conductivity test emerged during the cross examination of Professor Myerson. In light of the argument now advanced on this appeal, I must explain that evidence in a little detail. When asked about the shake test, Professor Myerson explained that he preferred to use a quantitative test, the easiest of which was to measure the conductivity of the solution as its concentration is increased. As this suggestion was explored with him, the following interchange took place (on day 5 at 632):
“Q. If it is a surfactant, it ought to form micelles and you can move straight to the determination of what the CMC is.
A. Assuming that you have found the condition and [it] did form micelles, then, yes, you would find the CMC, and that [it] was a surfactant at the same time.
Q. Right, and that would be a routine common general knowledge test at the date of the patents.
A. It certainly is a simple test that could be done in any reasonably equipped laboratory.
Q. I would just like you to agree with me yes or no that it was common general knowledge. It was, was it not?
A. To have it do the test? Is that the question?
Q. Yes.
A. Yes.”
38. A little later (on day 5 at 652) Professor Myerson provided this further elaboration in the context of Lin and Jiang:
“Q. That is a little bit circular, professor, is it not, because whether it is interesting or not depends in the first place on whether it is a surfactant and whether you can form micelles?
A. No, I guess my point is that I would not necessarily think that Lin & Jiang was a road you would want to go down just based on that paper, for various reasons I am sure we will discuss. But if you tell me to assume that I wanted to go down the road of Lin & Jiang; right, that is your assumption, then I certainly — the first step would be to find out if daptomycin formed micelles and at what conditions they formed, what conditions they could be de-micellised and whether daptomycin was stable in all those conditions, meaning it did not decompose.
Q. We will come on to this in the context of Lin & Jiang further, as you say, professor. To say there would not be any expectation to do the CMC test on daptomycin that it would not form micelles at a useful concentration, you have just got to test it and it is not a difficult test to do.
A. If you were interested in determining whether it formed micelles and what the CMC was, at a given set of conditions, you could certainly do the test — no question.
Q. By routine means?
A. As I said, a simple laboratory test.”
39. This was powerful evidence, but there was more when Mr Richard Meade QC, counsel for Hospira, returned to the topic (on day 6 at 696 to 697):
“A. As we have previously discussed, if you decide you wish to test something for surface activity and measure the CMC, that is a basic laboratory test that you can do.
Q. Right, so if you had a potential interest, if you read Lin & Jiang and thought, well, at least the first stage of that is good, daptomycin, I know at least it gets in the game because of its structure, you would not be put off from testing the CMC and the conditions at which you perform a CMC, because that is routine.
A. With that set of assumptions, if you said, I am interested in this, I want to see if daptomycin forms micelles, yes, you could go into the laboratory and do that test, I agree with that.
Q. And that would be routine and not unduly expensive or consuming of resources?
A. It is not a particularly difficult set of experiments to do.”
40. Mr Hinchliffe now submits that Professor Myerson was never asked about the conditions, in particular the pH conditions, under which such a test would be conducted. He continues that the pH conditions are important because Dr Kelleher gave clear evidence that daptomycin only forms micelles in very limited circumstances, in particular at low pH. He also submits that this evidence is confirmed by the teaching of the patent. It explains that daptomycin’s CMC at pH 4 is 400 µg/ml, but at pH 6.5 it is monomeric at 150mg/ml. So, argues Mr Hinchliffe, the skilled person carrying out such a test would have to pick a pH at which to carry it out and then would slowly increase the concentration of the daptomycin and determine the effect of that increase in concentration upon the conductivity of the solution. But unless the skilled person chose to perform this test at a particularly low pH, there was no evidence that micelles would form at any concentration. So the test would not reveal that it was a surfactant.
41. I cannot accept these submissions. First, the judge found on the evidence that it was entirely routine to study the effect of pH in a CMC study and he referred in this connection to the paper by D G Cooper and others entitled Enhanced Production of Surfactin from Bacillus subtilis by Continuous Product Removal and Metal Cation Additions, Applied and Environmental Microbiology, Sept 1981, 408-412. This describes a study of the effect of a change in pH on the surface tension of surfactin. Secondly, Professor Myerson did not at any stage suggest that the skilled person would only carry out a conductivity test at one concentration or at a single pH. Thirdly, Professor Myerson did not at any time suggest that daptomycin was so soluble that the skilled person would have had any difficulty finding its CMC at any reasonable concentration or pH. To the contrary, his evidence was to the effect that this would be entirely routine. Fourthly, it is apparent from Dr Kelleher’s evidence that Cubist looked at multiple pHs to determine the CMC of daptomycin. It found that micelles were formed at acidic pHs 2.5 to 5 and that it took the monomeric form at neutral pHs 6.0 to 7.5.
42. I am therefore satisfied that the judge was entitled to find that the skilled person would confirm that daptomycin was a surfactant by using routine and straightforward techniques, including shaking and conductivity testing.
Issue (ii) Control of micelle formation by pH adjustment
43. Cubist originally contended that the judge made two errors in reaching his conclusion that the skilled person would replace the methanol used to control micelle formation and dissociation in the process of Lin and Jiang with pH adjustment, namely (a) that he wrongly concluded that pH adjustment was a common general knowledge technique for altering the CMC of a surfactant; and (b) that he failed to consider whether the skilled person would have had the required fair expectation of success that pH adjustment would work as a way of adjusting the CMC. However, at the hearing of the appeal Mr Hinchliffe made clear that Cubist was not pursuing (a). It is therefore accepted for the purposes of this appeal that altering pH was a well-known technique for controlling micelle formation and dissociation.
44. Mr Hinchliffe has developed his argument on point (b) in the following way. He submits that the evidence did not establish and the judge did not find that the skilled team investigating the effect of a change in pH on the CMC would have had the necessary fair expectation of success. Indeed, the judge did not address the issue of expectation of success expressly in his judgment at all. Rather, he found the patented invention was obvious simply because adjustment of the pH would have been one of the avenues of investigation open to the skilled person seeking to replace the methanol used in the process described in Lin and Jiang. This was tantamount to a finding that including something in a research programme is enough to render it obvious and, as the Court of Appeal explained in Teva (UK) Ltd v Leo Pharma AS [2015] EWCA Civ 779, this is wrong in law and amounts to an error of principle.
45. Mr Hinchliffe continues that it was established on the evidence that if the skilled team found that the change in CMC on pH adjustment was very small, they would have thought it unlikely that altering pH would be a viable technique to use in place of the techniques disclosed in Lin and Jiang. Mr Hinchliffe supports this submission by pointing to the evidence of Dr Baker under cross examination. He contends Dr Baker accepted that, until the skilled team had tested the effect of a change in pH on the CMC of daptomycin, they would have had no way of knowing or predicting what it was; and that the team would have thought the change in CMC would have to be quite large for it to be of use. Mr Hinchliffe has also referred us to the evidence of Professor Myerson and submits that he too thought it would have been impossible to make a prediction about the effect of altering pH on the CMC before testing.
46. The upshot, says Mr Hinchliffe, is that a skilled person who had reached the stage of considering the alteration of pH (as opposed to one of the other options) to adjust the CMC would have had no expectation that the approach would work and would have been setting off on a research project of uncertain outcome. But when the judge conducted his assessment he failed to consider the skilled person’s expectation of success and had he done so he would or ought to have found that the path to the invention was not obvious.
47. There is a further limb to Mr Hinchliffe’s submissions. He also argues that the judge ought to have found that the skilled team would have been concerned that lowering the pH might adversely affect the stability of the daptomycin. In this connection Professor Myerson said in his second report that the skilled team would have been wary of developing any purification process which involved a low pH and leaving daptomycin in solution for any prolonged period of time. Further, Professor Myerson continued, ultrafiltration of large volumes of material on an industrial scale would take many hours. The team would therefore have had concerns that industrial scale purification of daptomycin using a pH of 4 would create a real risk of impurities being formed during the purification process.
48. Mr Hinchliffe has also taken us to the evidence of Dr Baker that the skilled team would have been astute to the need not to expose daptomycin to conditions likely to lead to its degradation. In this connection it was, says Mr Hinchliffe, common ground that the skilled team would have read a paper by L. E. Kirsch and others which shows that the formation of impurities is pH dependent and that the rate of impurity formation is highest at pH 4, that is to say the pH which the skilled team would adopt for daptomycin as part of a micelle formation strategy. Had the judge taken this into account as he should have done, he would or ought to have concluded that this would have lowered any expectation that pH might prove to be a viable replacement for the method described in Lin and Jiang.
49. Attractively though these submissions were presented, I cannot accept them. First, it is accepted by Cubist on this appeal that it was part of the common general knowledge of the skilled team that varying the pH of a lipopeptide biosurfactant would vary its propensity to exhibit surfactant properties including the formation of micelles.
50. Secondly and whilst it is true to say that Dr Baker accepted that, this being an empirical art, it would not have been possible to predict how much of an effect a change in pH would have on the CMC before testing, he maintained that the skilled person would nevertheless have considered it an obvious and attractive way to implement the teaching of Lin and Jiang and that the necessary testing would have been straightforward to carry out. In this connection, one of the particularly attractive features of the use of pH was the ability it conferred on the team to exert close control over the process.
51. Thirdly, Professor Myerson did not say in either of his reports that the skilled team would have had any concern that the effect of a change in pH on the CMC of daptomycin might not be sufficiently great to make it useful in a process of purification. Further, Professor Myerson accepted in cross examination that, had he been interested in reproducing the Lin and Jiang process for daptomycin, he would have looked at the three variables of pH, temperature and electrolyte; and that he would have appreciated that pH was “a good one” because it was controllable and required very little to be done to the solution.
52. Fourthly, the skilled team, considering the implementation of Lin and Jiang in relation to daptomycin, would have had a strong motivation to move away from methanol, would have known that altering pH was high up on a short list of alternative ways to change the CMC, and would have appreciated that the effect of altering the pH could be tested in a simple and straightforward way.
53. Fifthly, the judge was entitled to find that the skilled team would not have been deterred by the risk of degradation. Professor Myerson considered the question of obviousness on the assumption that one of the differences between the claimed invention and the prior art was that the invention was an industrial scale process as to size and yield. The scope of the claimed monopoly is not limited to industrial scale purification, however; and neither of the experts ever suggested that the skilled team would have been deterred from using pH as a way of controlling the CMC in a process for the purification of daptomycin on a laboratory or pilot scale, and both would have been within the scope of the claims.
54. Finally, there can be no doubt that the judge was aware that expectation of success may be a relevant matter to consider in assessing whether an invention was obvious. He expressly directed himself that where it is alleged that a step is obvious to try, the question is whether the skilled person would do so with a fair expectation of success, and that what amounts to a fair expectation of success will depend upon all of the circumstances of the case. Here he carried out his evaluation having regard to all of the evidence before him and all of the material circumstances.
Conclusion
55. I am satisfied that the judge had an ample evidential basis to find that the claimed invention was obvious in the light of Lin and Jiang and that his finding was properly reasoned. He has made no error of principle. I would dismiss this appeal.
Lord Justice Lewison:
56. I agree.
Leave a Reply