Neutral Citation Number: [2018] EWCA Civ 49
Case No: A3 2017 1483
IN THE COURT OF APPEAL (CIVIL DIVISION)
ON APPEAL FROM THE HIGH COURT OF JUSTICE
CHANCERY DIVISION
PATENTS COURT
Mr Justice Arnold
[2017] EWHC 987 (Pat)
Royal Courts of Justice
Strand, London, WC2A 2LL
Date: 25/01/2018
Before :
LORD JUSTICE LEWISON
LORD JUSTICE KITCHIN
and
LORD JUSTICE FLOYD
– – – – – – – – – – – – – – – – – – – – –
Between:
| (1) SANDOZ LIMITED (2) HEXAL AG |
Appellants | |
| – and – | ||
| (1) G.D. SEARLE LLC (2) JANSSEN SCIENCES IRELAND UC |
Respondents |
– – – – – – – – – – – – – – – – – – – – –
Charlotte May QC and William Duncan (instructed by Fieldfisher LLP) for the Appellants
Thomas Mitcheson QC and Stuart Baran (instructed by Bristows LLP) for the Respondents
Hearing date: 14 December 2017
– – – – – – – – – – – – – – – – – – – – –
Judgment Approved
Lord Justice Floyd:
- This appeal concerns the meaning of “the product is protected by a basic patent in force” in Article 3(a) of Regulation (EC) No 469/2009 concerning the supplementary protection certificate for medicinal products (“the SPC Regulation”). The SPC Regulation regulates the grant of supplementary protection certificates or SPCs which are instruments which, in effect, extend the term of granted patents beyond their originally allotted term in relation to a particular product which is the subject of a marketing authorisation. Their purpose is to compensate the patent proprietor for the time which it takes between filing a patent application and bringing a medicinal product to the market. This issue of interpretation of the SPC Regulation has been the subject of repeated references to the CJEU from the courts of the United Kingdom and elsewhere, without, thus far, the emergence of a clear legal criterion for national courts to apply in all cases.
- The first respondent is the proprietor (and the second respondent the exclusive licensee) of SPC/GB07/038 for a product described in the SPC as “Darunavir or the pharmaceutically acceptable salt, ester, or prodrug thereof”. The SPC covers a product marketed in Europe by companies associated with the second respondent under the trade mark “Prezista”. It is a protease inhibitor used in an anti-retroviral medication for the treatment of the HIV virus and AIDS. The respondents contend that the product described in the SPC was protected by European Patent (UK) No 0 810 209 (“the patent”) of which the first and second respondents were again, respectively, the proprietor and the exclusive licensee.
- The SPC will expire on 23 February 2019. The appellants brought the present proceedings in order to clear the way for the marketing of a generic darunavir product prior to the expiry of the SPC. The appellants’ product is not yet on the market. It is common ground, at least for the purposes of these proceedings, that the marketing of the appellants’ product would infringe the SPC, if the SPC is valid. The appellants contend that it is invalid because, on the true construction of Article 3(a) of the SPC Regulation, darunavir is not a product “protected” by the patent. There is no challenge to the validity of the patent itself.
- In a decision dated 3 May 2017, Arnold J decided that darunavir was a product protected by the patent. He declined to refer questions to the CJEU on the interpretation of Article 3(a) of the SPC Regulation because he considered that, on all tenable constructions of Article 3(a), darunavir was protected by the patent. This is an appeal from that decision and his consequent order.
- On the appeal the appellants were represented by Ms Charlotte May QC and Mr William Duncan. The respondents were represented by Mr Thomas Mitcheson QC and Mr Stuart Baran.
The SPC Regulation and the Explanatory Memorandum
- The SPC Regulation was preceded by the Commission’s Explanatory Memorandum COM 90 101 on the proposed Regulation (“the Memorandum”), a document which it is common ground is admissible as an interpretive aid to the Regulation. The Memorandum made the point that the Regulation was intended to be “simple and transparent”, not to “lead to excessive bureaucracy” and to be capable of implementation without “an excessive administrative burden being placed on” patent offices or the parties, or there being any requirement for the creation of a new administrative body. In particular, it was foreseen that “examination of the conditions to be fulfilled for the certificate to be granted involves the use of objective data that are easy to verify.”
- The Memorandum also made it clear that it was all types of research which were to be protected, not merely that part of the research programme which moved from the discovery of a structure-activity relationship to the individual compound: see e.g. paragraph 29:
“The proposal does not provide for any exclusions. In other words, all pharmaceutical research, provided that it leads to a new invention that can be patented, whether it concerns a new product, a new process for obtaining a new or known product, a new application of a new or known product or a new combination of substances containing a new or known product, must be encouraged, without any discrimination, and must be able to be given a supplementary certificate of protection provided that all of the conditions governing the application of the proposal for a Regulation are fulfilled.”
- The Memorandum suggests, at paragraph 39, and albeit in the context of Article 4, that a patent which protects a series of compounds based on a formula can be used as the basis for the SPC:
“It is thus often the case in the chemical and pharmaceutical field that a patent protects a series of products based on the same formula. However, only some of these products will subsequently be developed and possibly only one may be put on the market. In such a case, the certificate will only protect the product covered by the authorization and not all of the products protected by the patent.”
- The recitals of the SPC Regulation which are material are set out below:
[3] Medicinal products, especially those that are the result of long, costly research will not continue to be developed in the Community and in Europe unless they are covered by favourable rules that provide for sufficient protection to encourage such research.
[4] At the moment, the period that elapses between the filing of an application for a patent for a new medicinal product and authorisation to place the medicinal product on the market makes the period of effective protection under the patent insufficient to cover the investment put into the research.
[5] This situation leads to a lack of protection which penalises pharmaceutical research.
[6] There exists a risk of research centres situated in the Member States relocating to countries that offer greater protection.
[7] A uniform solution at Community level should be provided for, thereby preventing the heterogeneous development of national laws leading to further disparities which would be likely to create obstacles to the free movement of medicinal products within the Community and thus directly affect the functioning of the internal market.
[8] Therefore, the provision of a supplementary protection certificate granted, under the same conditions, by each of the Member States at the request of the holder of a national or European patent relating to a medicinal product for which marketing authorisation has been granted is necessary. A regulation is therefore the most appropriate legal instrument.
…
[10] All the interests at stake, including those of public health, in a sector as complex and sensitive as the pharmaceutical sector should nevertheless be taken into account. …
- Articles 1, 3, 4 and 5 of the SPC Regulation provide, so far as relevant:
Article 1
Definitions
For the purpose of this Regulation:
(a) ‘medicinal product’ means any substance or combination of substances presented for treating or preventing disease in human beings or animals and any substance or combination of substances which may be administered to human beings or animals with a view to making a medical diagnosis or to restoring, correcting or modifying physiological functions in humans or in animals;
(b) ‘product’ means the active ingredient or combination of active ingredients of a medicinal product;
(c) ‘basic patent’ means a patent which protects a product as such, a process to obtain a product or an application of a product, and which is designated by its holder for the purpose of the procedure for grant of a certificate;
…
Article 3
Conditions for obtaining a certificate
A certificate shall be granted if, in the Member State in which the application referred to in Article 7 is submitted and at the date of that application:
(a) the product is protected by a basic patent in force;
(b) a valid authorisation to place the product on the market as a medicinal product has been granted in accordance with Directive 2001/83/EC or Directive 2001/82/EC, as appropriate;
(c) the product has not already been the subject of a certificate;
(d) the authorisation referred to in point (b) is the first authorisation to place the product on the market as a medicinal product.
…
Article 4
Subject matter of protection
Within the limits of the protection conferred by the basic patent, the protection conferred by a certificate shall extend only to the product covered by the authorisation to place the corresponding medicinal product on the market and for any use of the product as a medicinal product that has been authorised before the expiry of the certificate.
Article 5
Effects of the certificate
Subject to the provisions of Article 4, the certificate shall confer the same rights as conferred by the basic patent and shall be subject to the same limitations and the same obligations.”
- It is common ground in the present case that the SPC satisfies the conditions specified in Article 3 (b), (c) and (d).
The patent
- The patent is entitled “Alpha- and beta-amino acid hydroxyethylamino sulfonamides useful as retroviral protease inhibitors”. It claims a priority date of 25 August 1992. The specification begins by stating at [0002] that the invention relates to such inhibitors, and in particular to “sulfonamide-containing hydroxyethylamine protease inhibitor compounds, a composition and the use thereof for preparing a medicament for inhibiting retroviral proteases such as human immunodeficiency virus (HIV) protease and for treating a retroviral infection e.g. an HIV infection”.
- The specification explains at [0003] that replication of a virus such as HIV involves a stage in which certain gene products are translated into proteins which are then processed by a retroviral protease. Inhibition of the retroviral protease may inhibit viral replication. After acknowledging certain prior art, the specification sets out a description of the invention at [0008]:
“The present invention is directed to virus inhibiting compounds and compositions. More particularly, the present invention is directed to retroviral protease inhibiting compounds and compositions, to the use of such compounds for preparing medicaments for inhibiting proteases, especially for inhibiting HIV protease and for treating a retroviral infection such as HIV infection and for treating AIDS, to processes for preparing the compounds and to intermediates useful in such processes. The subject compounds are characterized as sulfonamide-containing hydroxyethylamine inhibitor compounds.”
- The detailed description of the invention, starting at [0009], includes a series of paragraphs corresponding to the claims. These are framed by reference to two formulae, Formula I and Formula II. Formula I is shown below.
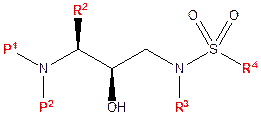
- Formula I identifies the essential backbone of the claimed class of compounds, and a number of variable substituents, shown as P1, P2, R2, R3 and R4. In addition to identifying the elements of structure, the formula shows a particular stereochemical orientation. The specification lists broad ranges of possible values of P1, P2, R2, R3 and R4. Preferred compounds of Formula I are described at [0010]-[0012], with increasing levels of specificity. 41 specific compounds are listed in [0012].
- Formula II is the same as Formula I except that it omits some of the stereochemistry. The specification again lists ranges of substituent groups and preferred compounds at [0013]-[0017].
- Prof Adlington, the appellants’ expert organic chemist, estimated that the number of compounds covered by claim 1 of the patent was somewhere between 7 x 10135 and 1 x 10377. There were something of the order of 8 x 1036 possibilities for the substituent P1 alone. By contrast the number of compounds specifically disclosed was, in his opinion, approximately 100. No attempt was made to challenge these numbers in the court below.
- It is explained at paragraph [0020] that compounds of the invention can be used to prepare pharmaceutical compositions useful for treating retroviral infections, in particular HIV or AIDS. The patent states at [0091] that the compounds of the invention are effective antiviral compounds and effective HIV protease inhibitors.
- It is common ground that there is no reference to darunavir anywhere in the specification.
- Claim 1 of the patent covers a class of compounds defined by means of Formula I. Claim 5 is an independent claim based on Formula II but with a narrower list of substituents. Claims 2, 10 and 11 contain narrower lists of substituents.
- The form of claim adopted in the present case is a familiar one in chemical patents. It is based on a structural formula having a fixed element with variable substituents to be chosen from amongst a defined class. Such a formula is known as a Markush formula, a term which, as the judge explained, is a term which originated in a 1924 decision of the Commissioner of Patents of the United States Patent and Trade Mark Office, ex parte Markush 1925 C.D. 126, 340 O.G. 839 (Comm’r Pat 1924). The Markush formula enables a large class of compounds to be claimed without the necessity of writing out every single chemical entity. The use of a Markush formula in a claim is an appropriate means of claiming an invention where the patentee’s invention has involved the discovery of a new technical effect which he predicts will be common to all members of the claimed class provided they share a common structural element (e.g. in the present case, the backbone structural element which is not permitted to vary in accordance with Formula I or II). Claims relying on a Markush formula to define their scope are referred to as Markush claims. They avoid the necessity of writing out in extenso every possible member of the claimed class. A danger with such claims is that they may cover compounds which do not show the claimed activity, and so result in insufficiency under Article 83 of the European Patent Convention (EPC), or equivalent national laws.
- The judge found that the practice of permitting the use of a Markush formula in a patent claim had been followed by patent offices worldwide, and in particular by the United Kingdom and the EPO. The UK Intellectual Property Office’s Manual of Patent Practice, says that Markush claims:
“… are often used in chemical cases as a way of setting out various functionally equivalent alternatives in one or more parts of the chemical compound being claimed”
Darunavir
- The structural formula of darunavir is shown below:
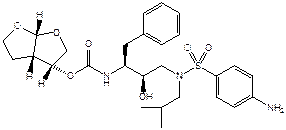
- The judge set out a helpful table to show how darunavir was a compound represented by Formula I and Formula II within claims 1, 2, 5, 10 and 11 in which the variable substituents were given values as follows:
| Group | Claim 1 | Claim 2 | Claim 5 | Claim 10 | Claim 11 | Darunavir |
| P1 | Heterocyclyl-oxycarbonyl | Heterocyclyl-oxycarbonyl | Heterocyclyl-oxycarbonyl | Heterocyclyl-oxycarbonyl | Heterocyclyl-oxycarbonyl | Bis-THF derivative |
| P2 | Hydrogen | Hydrogen | Hydrogen | Hydrogen | Hydrogen | Hydrogen |
| R2 | Aralkyl | Benzyl | Aralkyl | Aralkyl | Benzyl | Benzyl |
| R3 | Alkyl | Isobutyl | Alkyl | Alkyl | Isobutyl | Isobutyl |
| R4 | Aryl | Para-substituted aryl | Aryl | Aryl | Para-substituted aryl | Para-amino-phenyl |
- The appellants’ evidence was that the closest compound to darunavir disclosed in the patent was the seventh compound listed in paragraph [0012] (“Compound 7”). The difference between Compound 7 and darunavir is only in the P1 substituent, which, in the case of Compound 7 is an aralkoxycarbonyl group, specifically benzyloxycarbonyl. In darunavir the corresponding P1 substituent is a heterocyclyloxycarbonyl group, specifically a fused bis-tetrahydrofuran derivative. The difference in the two P1 substituents is illustrated below, with that for Compound 7 on the left and that for darunavir on the right:
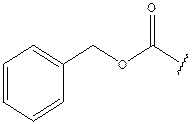
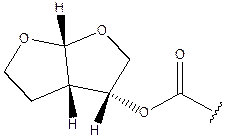
- The appellants’ evidence was that the heterocyclyloxycarbonyl substituent in darunavir was unusual, and not one which their expert had previously encountered. The appellants’ case is that such a substituent does not form part of the common general knowledge available to the skilled team at the priority date. The appellants’ evidence was that the structure of the P1 substituent group of darunavir was not published until after the priority date of the patent, in a paper by Arun K. Ghosh et al entitled “Structure-Based Design of HIV-1 Protease Inhibitors: Replacement of Two Amides and a 10π-Aromatic System by a Fused-Bistetrahydrofuran”, J. Med. Chem., 37, 2506-2508 (1994). So far as the position before the priority date was concerned, the respondents’ evidence drew attention to an article (M. Pezechk et al, “A new route to perhydro- and tetrahydro- furo-2,3b furans via radical cyclisation”, Tetr. Letters, 27, 32, 3715-3718 (1986)) which discloses the heterocyclyloxy portion of the P1 group in darunavir (but not the whole P1 group) as an intermediate in a reaction scheme for the synthesis of insect anti-feeding compounds.
The issue
- The appellants contend that for the product to be protected by a basic patent for the purposes of Article 3(a) it must be shown that “the skilled team would recognise the product as forming a part of the subject matter of the patent by reference to a careful reading of the patent based on the common general knowledge at the priority date”. They submit that, given the large number of compounds covered by the claim and the unusual nature of the P1 substituent on darunavir, that test is not satisfied in the present case. The respondents disagree and contend that darunavir will be protected by the patent if it is one of the class of products defined and claimed in the claims of the patent by reference to the Markush formulae.
The CJEU jurisprudence on the interpretation of Article 3(a)
- The approach to the interpretation of the predecessor to the SPC Regulation was stated by the CJEU in Case C-482/07 AHP Manufacturing v Bureau voor de Industriele Eigendom [2009] ECR I-7295 at [27]:
“Next, the Court observes that the second sentence of Article 3(2) of Regulation No 1610/96 must be interpreted not solely on the basis of its wording, but also in the light of the overall scheme and objectives of the system of which it is a part (see, by analogy, Case C-292/00 Davidoff [2003] ECR I-389, paragraph 24).”
- The SPC Regulation seeks to strike a balance between the interests at stake. These interests were identified by Advocate General Trstenjak in her opinion in Case C-130/11 Neurim Pharmaceuticals (1991) Ltd v Comptroller-General of Patents [EU:C:2012:268], [2013] RPC 23 as being (1) the undertakings which pursue costly pharmaceutical research, who favour an extension of the term of patent protection for the fruits of their research, (2) the producers of generic medicines who, as a consequence of the extended term are unable to produce and market generic medicines, (3) patients and the public who have an interest both in the introduction of new medicines and in those medicines being available at low prices and (4) State health systems which share the interest of the patient as well as an interest in preventing old active ingredients from being brought to the market in slightly modified form under the protection of certificates but without genuine innovation.
- To a patent lawyer, outside the context of the SPC system, the notion of what is protected by a patent is not a complex or difficult one. To answer the question of whether a particular product is protected, he or she will ask the question whether the product falls within the claims of the patent (applying Article 69 EPC and the Protocol). If so, then its sale or supply will be preventable by the patentee (as an infringing act applying the appropriate national rules for infringement). The product is therefore protected against sales by third parties. In conducting that exercise account is not normally taken of the presence of other active ingredients which are present in the accused product in addition to the patented one. Claims are normally interpreted as specifying what must be present, and as having nothing to say about what must not be present (although of course this can be made an express requirement of the claim). Further questions may arise if the doctrine of equivalents is invoked, but that problem does not arise on the present facts. It is tolerably clear that this straightforward approach is not correct in the context of the SPC Regulation. To understand the approach which must be taken, and the way it has been developed by the CJEU, it is necessary to track through a number of decisions of that court.
Farmitalia
- In Case C-392/97 Farmitalia Carlo Erba Srl [2000] RPC 580, Farmitalia had obtained a German patent for idarubicin. The claims of the patent specifically covered idarubicin hydrochloride. Farmitalia had also obtained a marketing authorisation for idarubicin hydrochloride and applied for a SPC for “idarubicin and salts thereof including idarubicin hydrochloride”. The German Patent Office granted a SPC for idarubicin hydrochloride, but refused to grant one for “idarubicin and salts thereof including idarubicin hydrochloride”. The Bundesgerichtshof (German Federal Court of Justice) referred questions concerning the interpretation of Article 3 of Council Regulation 1768/92/EEC (the predecessor to the SPC Regulation) to the Court of Justice. One question (its second) was as follows:
“According to which criteria is it to be determined whether the product is protected by a basic patent within the meaning of Article 3(a), where the grant of a protection certificate is sought for the free base of an active ingredient including any of its salts, but the basic patent in its patent claims mentions only the free base of this substance and, moreover, mentions only a single salt of this free base? Is the wording of the claim for the basic patent or the latter’s scope of protection the determining criterion?”
- The issue thus raised therefore concerned whether the inquiry as to what was protected was limited to what was “mentioned” in the claims, or whether one could also include that which would be within the scope of protection, such as alternative salts to the hydrochloride. The court’s answer was as follows:
“23. By its second question, the Bundesgerichtshof is, in substance, asking what are the criteria, according to Regulation No 1768/92, and in particular Article 3(a) thereof, for determining whether or not a product is protected by a basic patent.
…
- As Community law now stands, the provisions concerning patents have not yet been made the subject of harmonisation at Community level or of an approximation of laws.
- Accordingly, in the absence of Community harmonisation of patent law, the extent of patent protection can be determined only in the light of the non-Community rules which govern patents.
…
- The answer to be given to the second question must therefore be that, in order to determine, in connection with the application of Regulation No 1768/92 and, in particular, Article 3(a) thereof, whether a product is protected by a basic patent, reference must be made to the rules which govern that patent.”
- Had the matter been left there, one could have concluded that, so far as the UK is concerned, one would apply Article 69 EPC, if necessary the rules relating to infringing acts in section 60, and that EU law imposed no additional requirement. That, however, has subsequently proved not to be the case, although the nature of the additional requirement has proved somewhat elusive.
Medeva
- In Case C-322/10 Medeva BV v Comptroller-General of Patents, Designs and Trade Marks [2012] RPC 25, Medeva was the proprietor of a patent for the preparation of a combination of two antigens known as pertactin and FHA used in a vaccine against whooping cough. It was claimed that this combination produced a synergistic effect in vaccine potency. The claims covered the combination of pertactin and FHA. Medeva obtained marketing authorisations in respect of vaccines each of which was for immunisation against a number of diseases in addition to whooping cough, namely, diphtheria, tetanus, meningitis and polio. The vaccines contained between 8 and 11 different antigens, including, in each case, pertactin and FHA. Medeva filed five applications for SPCs in respect of the medicinal products the subject of the authorisations, all of which were refused by the UK Patent Office. The Comptroller refused four of the applications on the ground that they did not comply with Article 3(a) since the patent did not protect the combinations of antigens which were the subject of the authorisations and were specified in the applications. That decision was in line with a previous first instance decision of Jacob J in Takeda Chemical Industries Ltd’s SPC Applications (No 3) [2003] EWHC 649 (Pat); [2004] RPC 3.
- Medeva appealed to the Patents Court, but its appeal was dismissed by Kitchin J (as he then was), ([2010] EWHC 68 (Pat); [2010] RPC 20). Medeva appealed to the Court of Appeal, which referred the following five questions concerning Article 3(a) to the CJEU ([2010] EWCA Civ 700, [2010] RPC 27):
“1. Regulation 469/2009 (‘the Regulation’) recognises amongst the other purposes identified in the recitals, the need for the grant of an SPC by each of the Member States of the Community to holders of national or European patents to be under the same conditions, as indicated in recitals 7 and 8. In the absence of Community harmonisation of patent law, what is meant in Article 3(a) of the Regulation by ‘the product is protected by a basic patent in force’ and what are the criteria for deciding this?
- In a case like the present one involving a medicinal product comprising more than one active ingredient, are there further or different criteria for determining whether or not ‘the product is protected by a basic patent’ according to Article 3(a) of the Regulation and, if so, what are those further or different criteria?
- In a case like the present one involving a multi-disease vaccine, are there further or different criteria for determining whether or not ‘the product is protected by a basic patent’ according to Article 3(a) of the Regulation and, if so, what are those further or different criteria?
- For the purposes of Article 3(a), is a multi-disease vaccine comprising multiple antigens ‘protected by a basic patent’ if one antigen of the vaccine is ‘protected by the basic patent in force’?
- For the purposes of Article 3(a), is a multi-disease vaccine comprising multiple antigens ‘protected by a basic patent’ if all antigens directed against one disease are ‘protected by the basic patent in force’?” (emphasis added).
- Questions 1 and 2 are of particular importance in the present case. Question 1 was a version of the question asked in Farmitalia. Question 2, whether combinations are special, was new and was the first (but by no means the last) occasion on which this question had been put.
- In her opinion in Medeva Advocate General Trstenjak distinguished between what she called the “subject matter – or extent of protection” of the basic patent and its “protective effect”. At [67] she concluded that it was clear from the literal language of the Regulation (including Article 1(c) “product as such”) that:
“… a patent for ‘an’ active ingredient or ‘a’ combination of active ingredients which forms only part of the combination of active ingredients of a medicinal product cannot constitute a ‘basic’ patent within the meaning of Article 1(c) of [the SPC Regulation]. That is because on a literal interpretation, only the combination of active ingredients of that medicinal product in its entirety, and not the patented part of that combination, can be described as a product within the meaning of Article 1(b).”
- She continued at [68] and [69]:
“68. Nor is that conclusion altered in any way by the discussion conducted in the main proceedings in the context of Article 3(a) of Regulation No 469/2009, on the distinction between the subject‑matter – or extent of protection – and the protective effect of the basic patent. That debate concerns, in particular, the question whether the fact that an active ingredient which is the subject‑matter of a patent is an integral part of a combination of active ingredients and, as a consequence, that entire combination of active ingredients may not be produced or placed on the market without the consent of the patent proprietor (that is the protective effect of the patent) implies that the combination of active ingredients is deemed to be protected by a patent in force.
- The decisive consideration in that context is the fact that the definition of the basic patent in Article 1(c) of Regulation No 469/2009 takes as its basis the subject‑matter of the patent, and not its protective effect. A basic patent within the meaning of Regulation No 469/2009 must therefore be understood as one whose subject‑matter comprises either a product as such, a process to obtain a product or an application of a product within the meaning of Article 1(b) of Regulation No 469/2009.”
- This reasoning is important: the definition of “basic patent” in Article 1(c) is a patent which protects the product “as such”, and a product is the active ingredient or combination of ingredients of a medicine. It is comprehensible as a matter of language, therefore, that a patent for an active ingredient per se could not provide the basis for a SPC for a combination product, and a patent for a combination of two active ingredients could not provide the basis for a SPC for one active alone. In both cases there would be a mis-match and the patent would not protect the product “as such”.
- The Advocate General also conducted a teleological interpretation having regard to the various interests which I have already summarised at [29] above. She expressed a concern about the potential for abuse if combination SPCs could be obtained:
“96. If both the combination of active ingredients of a medicinal product and a patented active ingredient or combination of active ingredients contained in it could in future be classified as a product within the meaning of Article 1(b) of Regulation No 469/2009, there would be a risk that a manufacturer of medicinal products could develop a number of medicinal products with different combinations of active ingredients on the basis of one patented active ingredient or combination of active ingredients and place those products on the market with a time lag in some cases, for the purpose of optimising the protection under the certificate.
- An optimised duration – from the point of view of the manufacturer of medicinal products – of protection under the patent and the certificate could, for example, be achieved by ensuring that a first medicinal product with a patented active ingredient is placed on the market as quickly as possible in order to exploit the already existing patent protection commercially. Where the procedure for obtaining an authorisation to place the product on the market has taken longer than five years, the manufacturer of medicinal products could at the same time apply for a supplementary protection certificate and declare the complete combination of active ingredients as the product. He could then attempt to substantiate the protection under patent law for that product, required under Article 3(a) of Regulation No 469/2009, by reference to the protective effect of the basic patent for the patented active ingredient included in the combination of active ingredients. Subsequently, the manufacturer of medicinal products could place such products with slightly differing combinations of active ingredients, also including the patented active ingredient, on the market and, according to the same logic, apply for new supplementary protection certificates for them, which could then have a duration of up to five years.
- In order to prevent such an undermining of the system of limitation of the duration of the protection conferred by a certificate provided for in Regulation No 469/2009, Article 3(a) must be interpreted as meaning that the product within the meaning of that provision is the same as the product which forms the subject‑matter of the basic patent within the meaning of Article 1(c).
- The Advocate General’s answers to the questions referred were therefore:
“112. In order to answer the first question, as to how and on the basis of what criteria Article 3(a) of Regulation No 469/2009 is to be interpreted and applied, it is necessary to start from the principle that a product within the meaning of Article 3(a) is to be understood as a product which forms the subject-matter of a basic patent within the meaning of Article 1(c) of the regulation. Whether a product forms the subject-matter of a basic patent within the meaning of Article 1(c) and whether that product is protected by a basic patent in force in accordance with the requirement of Article 3(a) are determined, in principle, according to the rules governing the basic patent. However, the definition of a basic patent laid down in Article 1(c) of the regulation precludes combinations of active ingredients which are not the subject-matter of a basic patent, but nevertheless enjoy patent protection due to the presence of a patented active ingredient, from being characterised as a product within the meaning of Article 3(a).
- Against that background, the first question must be answered as follows: the condition for the classification of an active ingredient or combination of active ingredients of a medicinal product as a product within the meaning of Article 3(a) of Regulation No 469/2009 is that that active ingredient or combination of active ingredients forms the subject-matter of a basic patent within the meaning of Article 1(c) of that regulation. Whether an active ingredient or combination of active ingredients of a medicinal product forms the subject-matter of a basic patent within the meaning of Article 1(c) and whether that active ingredient or combination of active ingredients is protected by a basic patent in force in accordance with the requirement of Article 3(a) are determined, in principle, according to the rules governing the basic patent. However, the definition of the basic patent laid down in Article 1(c) of the regulation precludes use of the protective effect of the basic patent from being invoked as a criterion for the purpose of answering the question whether an active ingredient or combination of active ingredients of a medicinal product forms the subject-matter of a basic patent.”
- The Court of Justice also considered questions 1 to 5 together, choosing to re-phrase them as follows:
“19. By its first five questions, which it is appropriate to examine together, the Court of Appeal asks, in essence, whether Article 3(a) of Regulation No 469/2009 must be interpreted as precluding the competent industrial property office of a Member State from granting a SPC where the active ingredients specified in the application include active ingredients not mentioned in the wording of the claims of the basic patent relied on in support of such an application.
- While the Latvian, Lithuanian and Portuguese Governments submit that only the wording of the claims is relevant for the purpose of determining whether a product is protected by a basic patent in force within the meaning of Article 3(a) of Regulation No 469/2009, Medeva and the United Kingdom Government maintain that the concept of a ‘product … protected by a basic patent in force’ within the meaning of that provision corresponds to any combination of substances of a medicinal product directly infringing the patent.”
- The court then repeated what it had said in Farmitalia at [26]-[27] and continued:
“24. It should be noted that Regulation No 469/2009 establishes a uniform solution at European Union level by creating a SPC which may be obtained by the holder of a national or European patent under the same conditions in each Member State. It thus aims to prevent the heterogeneous development of national laws leading to further disparities which would be likely to create obstacles to the free movement of medicinal products within the European Union and thus directly affect the establishment and functioning of the internal market (see Case C 350/92 Spain v Council [1995] ECR I 1985, paragraphs 34 and 35; Case C 127/00 Hässle [2003] ECR I 14781, paragraph 37; and Case C 482/07 AHP Manufacturing [2009] ECR I 7295, paragraph 35).
- Moreover, it should be recalled that Article 5 of Regulation No 469/2009 provides that any SPC confers the same rights as conferred by the basic patent and is subject to the same limitations and the same obligations. It follows that Article 3(a) of the regulation precludes the grant of a SPC relating to active ingredients which are not specified in the wording of the claims of the basic patent.
- Similarly, if a patent claims that a product is composed of two active ingredients but does not make any claim in relation to one of those active ingredients individually, a SPC cannot be granted on the basis of such a patent for the one active ingredient considered in isolation.
- That approach is also borne out by the second subparagraph of paragraph 20 of the explanatory memorandum to the proposal for Council Regulation (EEC) of 11 April 1990 concerning the creation of a supplementary protection certificate for medicinal products (COM(90) 101 final) (‘the explanatory memorandum’), which, in so far as concerns what is ‘protected by the basic patent’, refers expressly and solely to the wording of the claims of the basic patent. That interpretation also accords with that given in recital 14 in the preamble to Regulation (EC) No 1610/96 of the European Parliament and of the Council of 23 July 1996 concerning the creation of a supplementary protection certificate for plant protection products (OJ 1996 L 198, p. 30), which refers to the need for ‘products’ to be ‘the subject of patents specifically covering them’.
- The answer to the first five questions is, therefore, that Article 3(a) of Regulation No 469/2009 must be interpreted as precluding the competent industrial property office of a Member State from granting a SPC relating to active ingredients which are not specified in the wording of the claims of the basic patent relied on in support of the SPC application.”
- The conclusion in [25] (and repeated in [28]) that Article 3(a) of the SPC Regulation precludes the grant of a SPC relating to active ingredients which are not specified in the wording of the claims of the basic patent is said to follow from Article 5, which provides that any SPC confers the same rights as conferred by the basic patent and possibly also from the need to avoid the heterogeneous development of national laws. The first of these considerations would seem to me to point in the direction of an infringement test, whilst the second would be satisfied by any test which the CJEU could lay down for application by all member states. Nevertheless the court’s conclusion is consistent with the reasoning of the Advocate General.
- When the case returned to the Court of Appeal, [2012] EWCA Civ 523; [2012] RPC 26, the obscurity of the court’s reasoning was the subject of argument and comment. In his judgment (with which Etherton and Elias LJJ agreed) Sir Andrew Morritt C recorded a submission of counsel at [28] that if the phrase “specified in the wording of the claims” in the Court of Justice’s decision was interpreted more narrowly so as to require the active ingredients to be expressly named then it would not be possible to grant SPCs in relation to “Markush” claims and other classes of product such as salts and antibodies as well as combination products generally.
- He went on to say, at [32], that it was nevertheless quite clear that the Court of Justice had rejected the infringement test. This was clear from the Advocate General’s opinion, and although the judgment of the court was “not so clear” the language used was inconsistent with any suggestion that the protective effect had any relevance to the issue before the court: see [32]. He continued at [33]:
“33.Thus the issue for the national court is to determine which active ingredients are specified in the wording of the claims. The ambit of “specified” may range from express naming, through description, necessary implication to reasonable interpretation. Where on that scale the dividing line is to be drawn will necessitate further references in due course in the light of the facts of the cases in which the issue arises. The problem for Medeva in this case is that wherever the dividing line is to be drawn the active ingredients relating to vaccines against diphtheria, tetanus, meningitis and polio are excluded.” (emphasis supplied).
Eli Lilly
- In Case C-493/12 Eli Lilly & Co Ltd v Human Genome Sciences Inc [2014] RPC 21, Human Genome Sciences (“HGS”) was the proprietor of a patent which disclosed the existence of a novel member of the TNF ligand superfamily of cytokines called Neutrokine-α. The patent disclosed the structure of Neutrokine-α, the sequence of its encoding DNA, its tissue distribution, its expression and the fact that it was a member of the superfamily. HGS had not found Neutrokine-α by traditional wet-lab techniques, but by “bio-informatics” or computational biology, i.e. computer-assisted sequence homology studies. Consequently, the description in the patent specification was not supported in any way by any data obtained from in vitro or in vivo studies, but was essentially a prediction based upon what was known about other members of the TNF superfamily. Claim 13 of the patent effectively covered any antibody that bound specifically to the full length Neutrokine-α polypeptide, or its extracellular domain of which there were potentially very large but in any event unknown numbers. It did not contain any structural definition or description of an antibody which might function as claimed.
- Eli Lilly had developed its own antibody for use in the treatment of autoimmune diseases, which it labelled LY2127399. LY2127399 bound specifically to Neutrokine-α, and in consequence would be an infringement of claim 13 of the HGS patent if the patent was valid and in force. Eli Lilly sought a declaration that any SPC that HGS might obtain based on Eli Lilly’s marketing authorisation for LY2123799 would be invalid. Its case was that the claims of the HGS patent were too broadly framed for it to be possible to regard LY2123799 as ‘specified’ in the wording of the claims as required by the Court of Justice’s decision in Medeva. In order for a SPC to be granted on the basis of HGS’ patent, its claims would have to be significantly more specific.
- Warren J referred the following questions to the CJEU:
“1. What are the criteria for deciding whether ‘the product is protected by a basic patent in force’ in Article 3(a) of [the SPC Regulation]?
- Are the criteria different where the product is not a combination product, and if so, what are the criteria?
- In the case of a claim to an antibody or class of antibodies, is it sufficient that the antibody or antibodies are defined in terms of their binding characteristics to a target protein, or is it necessary to provide a structural definition for the antibody or antibodies, and if so, how much?”
- Question 3 is new, the other questions having been asked and not answered either at all or in affirmative terms on a number of previous occasions. Question 3 raises the question whether a functional definition can ever “specify” the product. The CJEU gave its judgment without an Advocate General’s opinion. It dealt with all three questions together, which it reformulated as follows at [24]:
“By its three questions, which it is appropriate to consider together, the referring court asks, in essence, whether Article 3(a) of Regulation No 469/2009 must be interpreted as meaning that, in order for an active ingredient to be regarded as ‘protected by a basic patent in force’ within the meaning of that provision, the active ingredient must be identified in the claims of the patent by a structural formula, or whether the active ingredient may also be considered to be protected where it is covered by a functional formula in the patent claims.”
- The court recorded at [29] a submission by the Commission that to insist upon a literal reference to the active ingredient in the claims of a basic patent would be unduly restrictive. However the Commission’s view was that,
“…for a competent person and on the basis of the general knowledge of a person skilled in the art, it should be immediately evident from the claims of a basic patent that the active ingredient for which an SPC is sought is actually claimed by that patent.”
- The court repeated at [31] its previous observation in Medeva (at [23]) that since no harmonised European Union patent rules are applicable in the main proceedings, the extent of the protection conferred by a basic patent can be determined only in the light of the non‑European Union rules governing patents. It continued at [32]:
“32. It must be borne in mind that the rules for determining what is protected by a basic patent for the purpose of Article 3(a) of Regulation No 469/2009 are those relating to the extent of the invention covered by such a patent, such as the rules laid down in the main proceedings in section 125 of the UK Patents Act 1977. Where the patent in question has been granted by the EPO, those rules are also the rules laid down in the EPC and Protocol on the Interpretation of Article 69 of that convention.
- On the other hand, as is apparent from the response given by the Court to questions 1 to 5 in the case which gave rise to the judgment in Medeva, for the purpose of determining whether a product is ‘protected by a basic patent in force’ within the meaning of Article 3(a) of Regulation No 469/2009, recourse may not be had to the rules governing infringement proceedings, such as, in the main proceedings, those laid down in section 60 of the UK Patents Act 1977.
“34. By finding that Article 3(a) of Regulation No 469/2009 precludes the grant of an SPC relating to active ingredients which are not specified in the claims of a basic patent (see Medeva, paragraph 25, and the orders in Case C-630/10 University of Queensland and CSL [2011] ECR I 12231, paragraph 31, and Case C-6/11 Daiichi Sankyo [2011] ECR I 12255, paragraph 30), the Court emphasised the key role played by the claims for the purpose of determining whether a product is protected by a basic patent within the meaning of that provision.
…
- With regard to the fact that the marketing of that active ingredient by Eli Lilly during the lifetime of HGS’s patent would constitute an infringement of the patent, it is clear, in the light of what has been stated at paragraphs 32 and 33 above, that that is not a crucial factor, for the purpose of granting an SPC on the basis of Regulation No 469/2009, in particular Article 3(a) of that regulation, in the determination of whether that active ingredient is protected by that patent.”
- The court went on to consider the significance of the fact that LY2127399 was not mentioned in the patent, and said:
“38. It should be recalled that, in accordance with the case-law cited at paragraph 34 above, an active ingredient which is not identified in the claims of a basic patent by means of a structural, or indeed a functional definition cannot, in any event, be considered to be protected within the meaning of Article 3(a) of Regulation No 469/2009.
- With regard to the question whether the use of a functional definition may alone be sufficient, it should be noted that Article 3(a) of Regulation No 469/2009 does not, in principle, preclude an active ingredient which is given a functional definition in the claims of a patent issued by the EPO being regarded as protected by the patent, on condition that it is possible to reach the conclusion on the basis of those claims, interpreted inter alia in the light of the description of the invention, as required by Article 69 of the EPC and Protocol on the interpretation of that provision, that the claims relate, implicitly but necessarily and specifically, to the active ingredient in question.
- With regard to the requirements laid down by the EPC, it should, however, be noted that the Court does not have jurisdiction to interpret the provisions of that convention, since, unlike the Member States, the European Union has not acceded to the convention. The Court cannot, therefore, provide further guidance to the referring court concerning the manner in which it is to determine the extent of the claims of a patent issued by the EPO.
- Moreover, it should be recalled that the SPC is designed simply to re‑establish a sufficient period of effective protection of the basic patent by permitting the holder to enjoy an additional period of exclusivity on the expiry of that patent, which is intended to compensate, at least in part, for the delay to the commercial exploitation of his invention by reason of the time which has elapsed between the date on which the application for the patent was filed and the date on which the first MA in the European Union was granted (Case C-229/09 Hogan Lovells International [2010] ECR I-11335, paragraph 50; Case C-443/12 Actavis Group PTC and Actavis UK [2013] ECR, paragraph 31; and Case C‑484/12 Georgetown University [2013] ECR, paragraph 36).
42 As stated in recital 4 in the preamble to Regulation No 469/2009, the purpose of that additional period of exclusivity is to encourage research and, to that end, it is designed to ensure that the investments put into such research are covered.
- In the light of the objective of Regulation No 469/2009, the refusal of an SPC application for an active ingredient which is not specifically referred to by a patent issued by the EPO relied on in support of such an application may be justified – in circumstances such as those in the main proceedings and as observed by Eli Lilly – where the holder of the patent in question has failed to take any steps to carry out more in-depth research and identify his invention specifically, making it possible to ascertain clearly the active ingredient which may be commercially exploited in a medicinal product corresponding to the needs of certain patients. In such a situation, if an SPC were granted to the patent holder, even though – since he was not the holder of the MA granted for the medicinal product developed from the specifications of the source patent – that patent holder had not made any investment in research relating to that aspect of his original invention, that would undermine the objective of Regulation No 469/2009, as referred to in recital 4 in the preamble thereto.
- In the light of the foregoing considerations, the answer to the questions referred is that Article 3(a) of Regulation No 469/2009 must be interpreted as meaning that, in order for an active ingredient to be regarded as ‘protected by a basic patent in force’ within the meaning of that provision, it is not necessary for the active ingredient to be identified in the claims of the patent by a structural formula. Where the active ingredient is covered by a functional formula in the claims of a patent issued by the EPO, Article 3(a) of that regulation does not, in principle, preclude the grant of an SPC for that active ingredient, on condition that it is possible to reach the conclusion on the basis of those claims, interpreted inter alia in the light of the description of the invention, as required by Article 69 of the EPC and the Protocol on the interpretation of that provision, that the claims relate, implicitly but necessarily and specifically, to the active ingredient in question, which is a matter to be determined by the referring court.”
- Although it is the Court of Justice’s summary which is the binding source of law, for convenience I would shortly summarise the conclusions in Eli Lilly as follows:
- i) The rules for determining whether the product is protected are those relating to the extent of the invention (in the case of a European patent, those defined by Article 69 and the Protocol): [32].
- ii) Recourse may not be had to the rules relating to infringement, such as those in section 60 of the Patents Act 1977: [33].
iii) The fact that the product infringes is not, therefore, “a crucial” factor: [37].
- iv) The claims have a key role for the purpose of determining whether a product is protected by a basic patent within the meaning of Article 3(a): [34].
- v) An active ingredient which is not identified in the claims by any means (i.e. either a structural or functional definition) is not protected: [38].
- vi) It is not necessary for the active ingredient to be identified in the claims of the patent by a structural formula: a “functional formula” will do as well: [39], but:
vii) It must be possible to reach the conclusion on the basis of the claims, interpreted inter alia in the light of the description of the invention, that the claims relate, implicitly but necessarily and specifically, to the active ingredient in question: [39], [44].
viii) It is for the national court to determine the application of this test: [40], [44].
- When the Eli Lilly case returned before Warren J, [2014] EWHC 2404 (Pat); [2015] RPC 8, he did not find the application of the court’s reasoning to the facts of the case to be straightforward. In the end he considered that the claim “related to” Lilly’s antibody tabalumab. Lilly’s application for a declaration therefore failed.
- In his judgment in the present case Arnold J expressed disagreement with the route by which Warren J had come to this conclusion, but not necessarily with the result.
Two other cases
- It is necessary to mention two other cases, although these are not of such central relevance as Medeva and Eli Lilly. These are Case C-443/12 Actavis Group PTC EHF v Sanofi [2014] RPC 20, and Case C-577/13 Actavis Group PTC EHF and another v Boehringer Ingelheim Pharma GmbH & Co KG EU:C:2015:165.
- The court’s judgment in Actavis v Sanofi was released on the same day as its judgment in Eli Lilly. Sanofi was the proprietor of a patent for the drug irbesartan, an anti-hypertensive. It had been granted a SPC for irbesartan, optionally in the form of one of its salts. It had also been granted a SPC for a combination of irbesartan (again optionally in the form of a salt) in combination with another active, hydrochlorothiazide which was a diuretic. Claim 7 of the patent was to “irbesartan or one of its salts with acids or bases”, and claim 20 was to a pharmaceutical composition containing a composition in accordance with a preceding claim, in association with a diuretic.
- Actavis argued that the combination SPC was invalid on two grounds. Firstly they contended that the combination of irbesartan and hydrochlorothiazide was not protected by a basic patent within Article 3(a), since it was not specified or identified in the wording of the claims. Hydrochlorothiazide was not mentioned at all in the patent. Secondly they argued that the combination SPC was invalid because the product had already been the subject of the SPC for irbesartan. This offended against Article 3(c), or alternatively, it had been the subject of an earlier marketing authorisation namely the authorisation of the irbesartan itself, and therefore offended against Article 3(d).
- Arnold J referred the following two questions to the CJEU for a preliminary ruling:
“(1) What are the criteria for deciding whether “the product is protected by a basic patent in force” in Article 3(a) of … Regulation No 469/2009?
(2) In a situation in which multiple products are protected by a basic patent in force, does Regulation [No 469/2009], and in particular Article 3(c), preclude the proprietor of the patent being issued a certificate for each of the products protected?”
- The judge also proffered his own test “in the hope that it will assist the Court of Justice to provide a clear answer this time”. He asked himself what more it was necessary to show in addition to infringement in order for the product to be protected. He said at [76]:
“… the answer is that the product must infringe because it contains an active ingredient, or a combination of active ingredients, which embodies the inventive advance (or technical contribution) of the basic patent. Where the product is a combination of active ingredients, the combination, as distinct from one of them, must embody the inventive advance of the basic patent.”
- The court answered question 2 first, and therefore did not address question 1. It concluded that Article 3(c) operated to prevent the grant of a second SPC to Sanofi for the combination. The case is only of significance because of the court’s use, in connection with a statement of the objective of the SPC Regulation, of the concept of the “core inventive advance” of the patent. Thus at [41] the court says:
“It should be recalled that the basic objective of Regulation No 469/2009 is to compensate for the delay to the marketing of what constitutes the core inventive advance that is the subject of the basic patent, namely, in the main proceedings, irbesartan. In the light of the need, referred to in recital 10 in the preamble to that regulation, to take into account all the interests at stake, including those of public health, if it were accepted that all subsequent marketing of that active ingredient in conjunction with an unlimited number of other active ingredients, not protected as such by the basic patent but simply referred to in the wording of the claims of the patent in general terms, such as, in the case of the patent in the main proceedings, ‘beta-blocking compound’, ‘calcium antagonist’, ‘diuretic’, ‘non-steroidal anti-inflammatory’ or ‘tranquilizer’, conferred entitlement to multiple SPCs, that would be contrary to the requirement to balance the interests of the pharmaceutical industry and those of public health as regards the encouragement of research within the European Union by the use of SPCs” (emphasis added).
- In Actavis v Boehringer, Boehringer had a patent which claimed numerous molecules, including telmisartan and one of its salts. Telmisartan was an antihypertensive agent marketed by Boehringer and for which they obtained a SPC for “telmisartan optionally in the form of one of its salts”. Boehringer also later obtained a SPC for the combination of telmisartan and hydrochlorothiazide. In order to obtain this second SPC it had amended the basic patent to include a claim for telmisartan and hydrochlorothiazide. Birss J referred a number of specific and more general questions to the CJEU, not all of which the court felt it appropriate to answer. In short the court held that Articles 3(a) and 3(c) precluded the grant of a second SPC to Boehringer for the combination:
“36. In the light of the need, referred to, inter alia, in recital 10 in the preamble to Regulation No 469/2009, to take into account all the interests at stake, including those of public health, if it were accepted that all subsequent marketing of an active ingredient in conjunction with an unlimited number of other active ingredients which do not constitute the subject-matter of the invention covered by the basic patent would confer entitlement to multiple SPCs, that would be contrary to the requirement to balance the interests of the pharmaceutical industry and those of public health as regards the encouragement of research within the European Union by the use of SPCs (see, to that effect, judgment in Actavis Group PTC and Actavis UK, EU:C:2013:833, paragraph 41).
37 Accordingly, in view of the interests referred to in recitals 4, 5, 9 and 10 in the preamble to Directive 469/2009, it cannot be accepted that the holder of a basic patent in force may obtain a new SPC, potentially for a longer period of protection, each time he places on the market in a Member State a medicinal product containing, on the one hand, an active ingredient, protected as such by the holder’s basic patent and constituting the subject-matter of the invention covered by that patent, and, on the other, another substance which does not constitute the subject-matter of the invention covered by the basic patent (see, to that effect, judgment in Actavis Group PTC and Actavis UK, EU:C:2013:833, paragraph 30).
38 It follows that, in order for a basic patent to protect ‘as such’ an active ingredient within the meaning of Articles 1(c) and 3(a) of Regulation No 469/2009, that active ingredient must constitute the subject-matter of the invention covered by that patent.
39 In the light of the foregoing considerations, the answer to Questions 2 and 3 is that Article 3(a) and (c) of Regulation No 469/2009 must be interpreted as meaning that, where a basic patent includes a claim to a product comprising an active ingredient which constitutes the sole subject-matter of the invention, for which the holder of that patent has already obtained an SPC, as well as a subsequent claim to a product comprising a combination of that active ingredient and another substance, that provision precludes the holder from obtaining a second SPC for that combination.”
- The case therefore states the condition for a basic patent to protect the active ingredient as such as being that the active ingredient must constitute the subject matter of the invention covered by the patent. That test is the same as that advanced by the Advocate General in paragraph 113 of her opinion in Medeva.
Pending references
Teva v Gilead
- In Teva UK v Gilead Sciences Inc [2017] EWHC 13 (Pat), Arnold J made a further reference to the CJEU. In that case the SPC was for a combination product consisting of two active ingredients, namely (i) tenofovir disoproxil (“TD”) in the form of the fumarate (“TDF”) and (ii) emtricitabine (also known as FTC) in a single, fixed dose tablet. TD and emtricitabine were both inhibitors of a viral enzyme known as reverse transcriptase. Gilead’s patent claimed a class of compounds by reference to Markush formulae, which included TD. There was no reference in the patent to emtricitabine. It did however contain a subsidiary claim, claim 27, in the following terms:
“A pharmaceutical composition comprising a compound according to any one of claims 1-25 together with a pharmaceutically acceptable carrier and optionally other therapeutic ingredients.”
- Teva contended that the words “other therapeutic ingredients” did not specify any active ingredient, whether structurally, functionally or otherwise. On the contrary, they covered a virtually unlimited range of active ingredients for the treatment of many diseases. Indeed, emtricitabine was not approved for clinical use until seven years after the priority date of the patent and there was no evidence that it was known to be efficacious at that date. Gilead disagreed and contended that all that was necessary was that emtricitabine fell within the scope of protection of claim 27 of the patent applying Article 69 EPC and the Protocol.
- Both sides contended that the law was now sufficiently clear that a further reference to the CJEU was not necessary. The judge disagreed, and referred the following question to the CJEU:
“What are the criteria for deciding whether ‘the product is protected by a basic patent in force’ in Article 3(a) of the SPC Regulation?”
- Although the question is asked in general terms, it is plain from the arguments of the parties and the judgment that the uncertainty which gives rise to the need for a reference is the breadth of the definition of the “other therapeutic ingredients” in the claim, and whether such a broad definition can be sufficient for the purposes of Article 3(a). The judge also proffered again his own suggested test, first offered in Actavis v Sanofi (see paragraph [61] above) in case it might assist the CJEU to “to provide a clear answer this time”.
The German sitagliptin reference: Decision 14W (pat) 12/17
- Shortly before the hearing we were informed that, in Decision 14 W (pat) 12/17 dated 17 October 2017, the Bundespatentgericht (German Federal Patents Court, BPatG) had referred a question to the CJEU in a case which, like Eli Lilly, concerned a functional claim. The patent claim related to an “activity lowering effector [of DPP IV] for use in lowering the blood glucose level” in mammals. The invention was said to be useful in the treatment of diabetes. I will refer to this case as “Sitagliptin”.
- The active substance sitagliptin was developed after the date of the patent. The German Patent Office rejected the application for the SPC on the basis that the product was defined in the patent purely functionally, and that the subject matter of the patent did not extend to the subsequently developed product, sitagliptin. The appellant appealed on the basis that the contribution and core value of the patented contribution was not in specific compounds but in the use of DPP IV inhibitors generally. Sitagliptin was precisely such a DPP IV inhibitor and fulfilled the functional definition of the claimed class of active substances. An “individualised disclosure” was not necessary. It also relied on the decisions in Actavis v Sanofi and Actavis v Boehringer, as well as Arnold J’s “inventive advance” test as proposed in the present and earlier cases to support a submission that the essential question was whether the product in question is an embodiment of the inventive concept of the patent.
- The BPatG’s own view (see paragraph [3.3]) was that Actavis v Sanofi and Actavis v Boehringer did not affect the test set out in Eli Lilly. The court stated, at [4] and [5], its understanding of the case law of the CJEU in Medeva and Eli Lilly. At [5] it said that it considered decisive
“that the product in question is described in the claims of the basic patent in such a specific way that it forms part of the protected subject-matter of the patent claims. Article 69 is not only concerned with determining the extent of protection, but distinguishes between the determination of the subject-matter of the claims required in an initial examination step, on the one hand, and the determination of the extent of protection for this subject-matter as relevant to the question of infringement on the other hand”.
- The court considered (see paragraph [6]) that the requirements laid down by the Court of Justice were only met if the active ingredient in question “is specified in the claims in such a way that it can be identified as such and it is actually provided to the skilled person.” The court, at [8], expressly rejected the invitation to adopt a “core inventive contribution” test proffered in the cited English cases, which it considered had its place only in connection with Article 3(c). Perhaps more importantly, at [10], the court indicated that it considered the facts of the case now before us to be “absolutely comparable to those in the present case”:
“This is because Darunavir, the substance at issue in the British decision, (like an almost endless number of other compounds) falls under a Markush formula contained in the claims of the basic patent, while in the present case the active substance sitagliptin (like an incalculable number of other compounds), falls under a functional definition which is contained in the patent claims.”
- Accordingly the BPatGhas decided to refer questions to the CJEU. One of the reasons it chose to do so was the existence of a differing proposed test in the United Kingdom, as well as a divergence of practice at the level of the granting authorities in other member states. The court’s questions are:
“1. Is a product protected by a basic patent in force according to Article 3(a) of [the SPC Regulation] only if it belongs to the protected subject-matter as defined by the patent claims and is thus provided to the person skilled in the art as a specific embodiment?
- Is it therefore not sufficient for the requirements of Article 3(a) of [the SPC Regulation] that the product in question meets the general functional definition of an active substance class as mentioned in the claims, but beyond this does not constitute a specific embodiment of the method protected by the basic patent?
- Is a product consequently not protected under Article 3(a) of [the SPC Regulation] by a basic patent in force even if it is covered by the functional definition contained in the claims, but was developed based on independent inventive activity only after the basic patent application was filed?”
- The first two questions are, in effect, based on the theory that the active ingredient must be provided to the skilled person as a specific embodiment. That theory appears, in effect, to demand an actual disclosure of the active ingredient.
The judgment of Arnold J
- The judge dealt with the law relatively briefly, as he had previously summarised it in Teva UK v Gilead (cited above). The judge observed at [61] that the broadest tenable interpretation of Article 3(a) was that it was sufficient that the product fell within at least one claim of the patent applying the rules relating to the extent of protection as ascertained by the application, in the case of a European patent, of Article 69 EPC and the Protocol. He recognised, however, that at present it did not appear that that interpretation was correct, because the CJEU has so far held that more is required.
- The CJEU jurisprudence had initially indicated that it was necessary for the product to be “specified” or “identified” in the wording of the claims. Subsequently, however, the CJEU had held that it was not necessary for the active ingredient to be identified in the claim by means of a structural formula: and that it was sufficient for the active ingredient to be covered by a functional description provided that the claims “relate, implicitly but necessarily and specifically, to the active ingredient”. It was clear from this that the identification of the active ingredient in the claim by means of a structural formula is permissible, but not essential; that it was not necessary for the claim individually to name or depict the active ingredient; and that it was not necessarily an objection that the claim in question covers a large number of other compounds in addition to the active ingredient in question.
- For those reasons the judge held at [64] that it was sufficient for the claim to “specify the product by means of a Markush formula which covers it” (at least without resort to equivalents). On that basis darunavir was “protected” by the patent.
- The judge said that he nevertheless remained of the view which he had expressed in both Actavis v Sanofi and Teva v Gilead that a better test would be one which requires that the product fall within the claim and that it should embody the inventive advance (or technical contribution) of the claim. If that test was applied to the facts of the present case, the answer was clear. The inventive advance (or technical contribution) of claim 1 of the patent lay in the identification of the compounds covered by claim 1 as having utility as HIV protease inhibitors. Darunavir embodies that inventive advance.
- On all tests considered thus far, darunavir was a product protected by the patent. The judge turned to the appellants’ test at [67]. As to that he made two points of importance for present purposes. Firstly, the test was really a breadth of claim test. It was not the function of patent offices when assessing applications for the grant of SPCs to have to consider whether the breadth of the claims of the basic patents relied on is justified. That would not make for a simple and transparent system, as envisaged in paragraph 16 of the Memorandum. By contrast, paragraph 39 of the Memorandum did envisage SPCs being granted where “a patent protects a series of products based on the same formula”. Secondly he said that it was implicit in the appellants’ case that some compounds covered by claim 1 of the patent were “protected” by the patent, while others were not; but it was wholly unclear where and how the line between the two groups of compounds was to be drawn.
- The judge considered that, in reality, the Claimants’ objection was that the claims of the patent were of excessive breadth because they encompassed a vast number of compounds, of which the skilled person could not make even a tiny fraction, and which it is not plausible would all be efficacious as protease inhibitors. If well founded, however, that was an objection to the validity of the patent. It amounted to saying that the claims were obvious on AgrEvo grounds or insufficient. However, the appellants had not put the validity of the patent (as opposed to the SPC) in issue, and this collateral means of challenging validity was not open to them.
- The judge therefore held that there was no tenable construction of Article 3(a) which led to the conclusion that darunavir was not protected by the patent.
The arguments on the appeal
- Ms May submitted, firstly, that the judge had made “a fundamental error of principle” in not referring questions to the Court of Justice. In her written submissions she said it simply was not open to the judge not to refer the matter to the CJEU. I do not think this is a productive line of argument, however. The real question for us is whether the judge’s self-direction on the applicable law is correct, and whether he then applied the law correctly to the facts. If he did so the appeal will fail. If he did not do so, we can either allow the appeal, or if the law is uncertain, refer a question ourselves to the CJEU. The judge’s decision to refer or not to refer does not give rise to an independent ground of appeal.
- The respondents submitted that a reference would be futile, given that there is no real possibility of the answer to such a reference being received and dealt with in this court before the expiry of the SPC. They did not, however, submit that the appeal was otherwise an abuse of process, on the grounds, for example, that it was now wholly academic. It follows that we are obliged to engage with the issues on the appeal. If, we consider that a reference to the CJEU is necessary to enable us to decide those issues, it must follow that a reference is not futile.
- More significantly, Ms May submitted that the judge had wrongly analysed the Medeva and Eli Lilly decisions. Medeva had laid down a general test for when an active ingredient was protected by a basic patent: it had to be specified in the wording of the claim. That test applied to combination claims as well as to claims for a single class of active ingredients. It had, however, given rise to the question of how specific the claim must be. The Eli Lilly case had attempted to answer that question, but had not provided a complete answer. The requirement that the claims relate “implicitly, but necessarily and specifically” to the product applied to Markush claims but remained unclear. The judge had failed to give weight in his analysis of the Eli Lilly judgment to the need for the claim to relate to the active ingredient “necessarily and specifically”. The first half of paragraph [43] of the Eli Lilly judgment contained an indication as to what was meant by this phrase, by indicating that it was relevant if the patentee had not carried out “more in-depth research” to “identify his invention specifically, making it possible to ascertain clearly the active ingredient which may be commercially exploited in a medicinal product corresponding to the needs of certain patients”.
- Ms May submitted that, if the judge had grappled with the Eli Lilly test, he should have concluded that, because of the unusual nature of the P1 substituent group, darunavir was not specified implicitly, but necessarily and specifically in the claims of the patent. Darunavir was just one of a vast number of compounds encompassed by the claims of the patent. Further, even if one could contemplate writing down the entire list, darunavir would not be on it because the specific P1 group employed in darunavir was not part of the common general knowledge of the skilled person.
- Ms May next submitted that the judge’s inventive advance test was wrong in principle, at least when applied to a Markush claim. In the case of a Markush claim, the default position was that every compound which fell within the formula in terms of its structure would also have the claimed activity, and therefore embody the inventive advance. The inventive advance test therefore added nothing to an infringement test in the case of this particular class of claim. Yet the CJEU had held that “something more” than infringement was required before the product could be deemed protected by the basic patent.
- Ms May also took issue with the way the judge applied the inventive advance test to the facts of this case. There was evidence in the case, which the judge treated as irrelevant, that some of the compounds covered by the claim were difficult to synthesise or unstable. Accordingly this was a case where the inventive contribution was not commensurate with the breadth of the Markush claim. Ms May went further and invited us to make a finding that darunavir did not fall within the inventive contribution of the patent, because of the unusual nature of the P1 group. A product which the skilled person cannot identify from the patent cannot be taken to be part of the inventive advance of that patent.
- Finally, Ms May submitted that the judge had failed to adopt the appellants’ test, which was a test that struck a fair balance between the extremes of the infringement test on the one hand and a disclosure test on the other. The appellants’ test gave rise to a protected class of products which was narrower than the scope of the claim, because it was limited to the products which the skilled person could envisage based on the common general knowledge. It was not, however, as narrow as a specific disclosure test, which Ms May disclaimed before us, as she had done before the judge. The correct approach was to tell the skilled person the structure of the product which is to be the subject of the SPC, and to ask whether that product was one which he or she could have identified at the priority date from a careful reading of the patent using common general knowledge.
- Mr Mitcheson submitted that the appellants’ test was unworkable, and contrary to the simple, transparent, easy to administer and objective test foreseen in the Memorandum. Instead of the relatively straightforward test of whether the Markush formula encompassed the product in question, it required national patent offices to conduct an evidence-based enquiry into whether the substituent groups not actually disclosed in the specification were or were not part of the common general knowledge. That was not a test which had thus far been adopted in relation to Markush claims. Indeed the SPC for darunavir had been granted in all 16 designated states of the patent, including Germany.
- Mr Mitcheson also submitted that the effect of the test propounded by the appellants was to discriminate against a phase of medical research, namely the phase in which the structure-activity relationship has been discovered but the individual specific compound which was to be administered to humans had not yet been singled out. This was contrary to the objectives spelled out in the Memorandum that all types of medical research were to be incentivised.
- Mr Mitcheson identified what he called a spectrum of specificity. In Medeva there was nothing at all in the claim to identify the additional ingredient, beyond the use of the word “comprising” which allowed for additional ingredients for the purposes of determining infringement. Then there were claims which actually called for “other therapeutic ingredients”, such as that in Teva v Gilead. That was still an entirely generic description, but it was a suitable candidate for a reference to the CJEU because it was more specific than Medeva. Then, in Eli Lilly, the claim had a functional definition of an antibody. It was plain that this definition did not, indeed could not, define the antibody structure precisely, but the CJEU did not reject this mode of definition outright. The national court had subsequently upheld the validity of the SPC. Finally there was a claim of the kind which was the subject of the Sitagliptin reference. That claim was also functional, but did not tell you whether the “effector” was an antibody, a small molecule or a biomolecule. This was less defined than the claim in Eli Lilly, which made a reference an appropriate course.
- The present case did not throw up the problems of specificity which arose in Eli Lilly and in the German reference (which were both functional claims, the German claim being even less specific than the claim in Eli Lilly). The present case defined a class of compounds by both function and structure. It was not necessary in a case such as the present, where the active ingredient was defined in the claims by means of a structural formula, to look for further specificity in the claims. There was no unanswered question which had to be referred to the CJEU. That was illustrated by the fact that a number of national offices had rejected the SPC which was the subject of the Sitagliptin reference, whilst all national offices asked to do so had granted the daunavir SPC the subject of the present case.
- So far as the Sitagliptin reference was concerned, Mr Mitcheson submitted that the German court had misunderstood the basis on which Arnold J had decided the present case. The court appeared to have understood that Arnold J had decided the case on the basis of the core inventive advance test. That was not the case, however. The ratio of Arnold J’s decision was that there was no tenable test on which darunavir could be held not to be protected by the patent. Further, it was not the case at all, as the German court had suggested, that the facts of the two cases were indistinguishable.
- Paragraph [43] of the Eli Lilly decision was concerned with the situation where a third party had obtained a marketing authorisation for a specific product not mentioned in the basic patent. That had originally been an issue in the reference, and was therefore the subject of evidence and submissions, but Eli Lilly had dropped it before the hearing. Moreover in the Eli Lilly case the CJEU must have implicitly rejected Lilly’s case that the criteria for determining whether a product is protected involved inquiring into the breadth of the claim. That was effectively the same test as was now propounded by the appellants here.
- Mr Mitcheson further submitted that, although it was correct that the Markush formula in the present case defined a large number of compounds, the presence of the mandatory structural backbone in fact meant that it defined a fairly limited chemical space.
- Mr Mitcheson submitted, finally, that the problem of abuse identified by the Advocate General in Medeva concerning the grant of successive SPCs for a patented active ingredient in combination with a variety of other ingredients had been dealt with by Article 3(c) in Actavis v Sanofi and Actavis v Boehringer.
Discussion
- I will refer to the CJEU’s requirement, formulated for the first time in Eli Lilly, that, in order to be protected by the basic patent, the claim must relate to the product implicitly, but necessarily and specifically as “the Lilly requirement”. If it were possible to say that the Lilly requirement is limited to functional claims, or alternatively, if it applies to all claims, that it is always satisfied by a Markush claim which covers the active ingredient, then it would follow that the appeal must be dismissed. If the position is not clear, however, we may have to refer a question to the CJEU.
- An important point of detail is the time at which and the circumstances in which the national authority has to determine whether a product is protected by a basic patent. The appellants do not suggest that the exercise for the skilled person of determining whether a product is protected by a basic patent should be performed in ignorance of the product in question. The judge accepted the respondents’ submission that the question whether a product is protected by claims in a basic patent falls to be judged when the product is known, and when it has been authorised to be placed on the market as a medicinal product. I did not understand Ms May to challenge that proposition. I consider it to be correct. That conclusion still leaves open the question of what is the necessary exercise for determining whether the product is protected by the patent. The two candidates which remain are (a) asking whether it is clear that the product is claimed as such; and (b) asking whether the product is one which is sufficiently identified. A test which goes further and asks whether the active ingredient is specifically disclosed is not advanced by the appellants.
- One would have thought that the choice between these two remaining alternatives could be made according to what it is that the Lilly requirement is seeking to achieve. If the object of the requirement is to ensure that the product is an embodiment of the inventive effort or advance contributed by the patentee, then the first of the two candidates would seem to be the appropriate test. On the other hand, if the object of the requirement is to ensure that the patent demonstrates that the proprietor has in fact come close to an actual realisation of the product, then the second of the two candidate tests would seem to be more appropriate. Indeed one could go further and insist on an actual disclosure of the active ingredient in question. Even then, given the ability to generate chemical structures by computer technology without wet-lab techniques, the mere fact that a structure is precisely specified in a patent may be a poor indicator of whether the patentee has in fact actually made it or performed any research on it.
- The Lilly requirement stems from the CJEU’s decision in Medeva that all members of a combination of active ingredients which is the subject of a SPC must be specified in the wording of the claims. The requirement follows, at least according to the Advocate General’s reasoning, from the fact that the product the subject of the SPC is to be protected “as such” by the basic patent. Medeva was not a case about how closely each component of the combination needs to be specified in the claims. Rather it was a case which ruled out a SPC where some of the products were not specified in the claims at all. It was a case in which one had a plain mis-match between the basic patent and the SPC. If there is a mis-match between the product the subject of the SPC and that protected as such by the patent, one can see how infringement rules could lead to an incorrect result. That is because the infringement rules do not require matching in this way. A claim to a single active ingredient is infringed by a combination of that product and another active: but the patent does not protect the combination product as such.
- In the case of a SPC with a single active ingredient, the reasoning in Medeva requires that the basic patent protect that active ingredient as such. The reasoning is not informative as to how specifically the claims must focus on the active ingredient, or what underlies the requirement that they should do so.
- It is not clear to me that the CJEU’s judgment in Eli Lilly takes the matter much further. Eli Lilly was specifically concerned with functional claims. Functional claims and structural claims are fundamentally different in terms of what they require the skilled person to do in order to determine whether a particular product is specified by a claim. A structural claim simply requires one to read the claim and the specification, look at the structure of the product and decide whether it is a product specified in the claims. Functional claims, by contrast, require one to perform a functional test on the product. For example a claim to an antibody by reference to a binding ability normally requires one to perform a practical test to determine whether the antibody actually binds to something else.
- Medeva therefore left a substantial unanswered question as to whether a product could be specified by a functional claim at all. It might be thought that it was simply too difficult for a patent office, operating the simple, transparent and objective system set up by the SPC Regulation, to work out whether a particular antibody was one specified in the claim. Problems of that nature do not arise in the case of Markush claims.
- The submission by the Commission recorded at paragraph 29 of the judgment in Eli Lilly (see paragraph [51] above) that “on the basis of the general knowledge of a person skilled in the art, it should be immediately evident from the claims of the basic patent, that the active ingredient for which a SPC is sought is actually claimed by that patent” can also be seen to be directed at the problem of linking a functional limitation in a claim to a given product. The Commission was not necessarily urging the court to impose a new standard of specificity in claims which already define a class of compounds by reference to a Markush formula. Indeed the Commission accepted that a literal reference was not required. The submission is expressed in language (“evident from the claims of a basic patent that the active ingredient … is actually claimed”) which is consistent with the first of the candidate tests I have outlined above.
- Such help as the judgment in Eli Lilly gives as to what underlies the specificity requirement is to be found, not in its core reasoning, but in paragraph [43] of the judgment. That paragraph appears to be one designed to give the national court assistance in arriving at its judgment in the main proceedings. It is true that that paragraph is in the context, additionally, of an application for a SPC based on a third party’s marketing authorisation. But the first part of the paragraph seems to me to indicate, albeit without great clarity, that the court considers that at least one way of preventing or hindering the marketing authorisations of third parties from being used as the basis for SPCs is to insist on a high degree of specificity in the basic patent. That might help to prevent a patentee spreading the net in his patent claims widely and unspecifically, and subsequently fastening on a competitor’s successfully marketed drug to obtain an extended term which he has not earned. That is a consideration which does not only arise in the context of functional claims, and lends force to the suggestion that the requirement for a high degree of specificity is a general one.
- If it is right that there is a general requirement that the active ingredient which is the subject of the SPC must be identified, the question arises of how specific the claims must be. I agree with Mr Mitcheson that there is a spectrum of specificity indicated by the factual scenarios in the various decided cases and references. I would regard it as plain that a Markush claim can in some circumstances amount to a sufficiently precise claim for the purposes of Article 3(a), for example where individual substituents are identified in the specification, or where classes of such substituents are set out, and the skilled person would be able to determine the extent of those classes. However I do not think one can extract from the reasoning in Eli Lilly the proposition that an active ingredient is adequately identified by a Markush formula however broadly that formula is framed and however obscure the particular substituent required to form the active ingredient the subject of the SPC. I think it is at least arguable that that substituent must be amongst those which the skilled person would be able to identify based on his common general knowledge at the priority date. I say so for two reasons.
- My first reason for considering that proposition to be arguable is the insight which paragraph [43] of Eli Lilly gives into the CJEU’s thinking concerning the purpose of the requirement for the active ingredient to be identified. If the objective is to ensure that the patent proprietor has come close to an actual realisation of the product, then the fact that the relevant substituents cannot be arrived at from a reading of the specification and the common general knowledge may be highly relevant.
- My second reason for considering that proposition to be arguable is the view expressed by the BPatG in the Sitagliptin reference that the functional formula in that case and the Markush formula in the present case are factually indistinguishable for the purposes of Article 3(a). Whilst I consider that there are significant points of distinction relevant to Article 3(a) between the two classes of case, the BPatG does not agree. It would therefore appear likely that a German court would take the view that a Markush formula may, at least in a case like the present, fail to provide protection within the meaning of Article 3(a). A decision by this court that a Markush formula will always be adequate for that purpose would therefore lead to conflicting decisions at least in these two member states.
- Like the judge, however, I am concerned with what I see as a fundamental defect with the “identification” test. The CJEU jurisprudence to date seems to take it as read that a claim can identify active ingredients with specificity. However that is not the function of claims in patents. Instead, claims are concerned with setting the limits to the monopoly. A further defect of the focus on the claim is that claims can be manipulated by skilful drafting to protect combinations, without distinguishing between genuine combinations of products which work together in a new and advantageous way so as to constitute an inventive advance, and mere collocations of products giving rise to their separate individual effects. I agree with the judge that a far better test would be to ask whether the product the subject of the SPC embodies the core inventive advance of the basic patent.
- I think Ms May is wrong when she submits that the core inventive advance test is inadequate because it imposes no greater requirement in the case of a Markush claim than would be imposed by an infringement test. That submission is based on the false premise that any test one proposes, when applied to a particular type of claim, must add something to a test of infringement. I do not follow why that should be so. If the objective behind the Lilly requirement is understood to be that the active ingredient must embody the inventive advance, then that objective is satisfied by a valid Markush claim. Every compound encompassed by the claim delivers the core inventive advance. In other types of claim the test will not be satisfied. To take an example based on the facts of Medeva, if the vaccine the subject of the SPC did not take advantage of the synergistic effect in vaccine potency of the combination, for example by using normal doses, then it would not embody the core inventive advance.
- The adoption of the core inventive advance test remains a possibility given the pending references from in Sitagliptin and Teva v Gilead, and the fact that it is becoming clear (see Actavis v Novartis, Actavis v Boehringer) that the possible abuse identified by the Advocate General in Medeva can be dealt with through Article 3(c). If that test were adopted across the board and applied here, despite Ms May’s submissions concerning its application (which I reject), I have no doubt that the SPC would satisfy Article 3(a).
- By recognising the common general knowledge test as arguable I am not to be taken as suggesting that there are not some very undesirable consequences if this approach were to be adopted. Principal amongst these is the difficulty the test would present to patent offices around Europe in its application. It is a very long way indeed from the simple, transparent and objective approach foreseen by the Memorandum. I also agree with Mr Mitcheson that there is a danger that too narrow an approach to what is protected will discriminate unfairly against certain stages of pharmaceutical research, contrary to the objectives in the memorandum, although it might fairly be said that this raises an issue of policy.
- I do not, however, accept that the appellants’ common general knowledge test is essentially a breadth of claim test, if by that it is meant that it is a collateral attack on the validity of the patent. It is simply a test of whether the claim meets the requirement that the active ingredient be identified specifically.
- In case it would assist the Court of Justice I will express my provisional conclusion. Left to myself, I would have concluded that darunavir was a product protected by the claims of the patent. In the case of a product with a single active ingredient and a patent with a claim which identifies a number of compounds by means of a Markush formula, all of which compounds embody the core inventive technical advance of the patent, the test should be whether the skilled person, considering the claims of the patent on the one hand and the structure of the product in question on the other, would immediately recognise that the active ingredient in question is one of those specified by the formula. On the facts of the present case as found by the judge, that test is satisfied. However, for the reasons I have given, it is not clear that this is the correct approach in EU law.
- I would therefore propose that this court should stay the present appeal proceedings and refer the following question to the CJEU:
“Where the sole active ingredient the subject of a supplementary protection certificate issued under [the SPC Regulation] is a member of a class of compounds which fall within a Markush definition in a claim of the patent, all of which class members embody the core inventive technical advance of the patent, is it sufficient for the purposes of Article 3(a) of the SPC Regulation that the compound would, upon examination of its structure, immediately be recognised as one which falls within the class (and therefore would be protected by the patent as a matter of national patent law) or must the specific substituents necessary to form the active ingredient be amongst those which the skilled person could derive, based on their common general knowledge, from a reading of the patent claims?”
- If my Lords agree I would invite the parties to seek to agree a draft order for reference. In the event that they cannot agree we will give directions for submissions in writing.
Lord Justice Kitchin
- I agree.
Lord Justice Lewison
- I also agree.
Leave a Reply